2727
Retrospective Motion Correction of multi-shot 3D GRASE Arterial Spin Labelling using ESPIRiT reconstruction1UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2School of Biomedical Engineering and Imaging Sciences, Kings College London, London, United Kingdom, 3Dementia Research Centre, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 4Neuroradiological Academic Unit, Department of Brain Repair and Rehabilitation, University College London, London, United Kingdom, 5Wellcome Centre for Human Neuroimaging, University College London, London, United Kingdom
Synopsis
A retrospective motion correction method is presented, for multi-shot 3D GRASE ASL. ESPIRiT is used to calculate coil sensitivity fields, using raw ASL k-space data. These are used to reconstruct complete images from the interleaved fractions of k-space sampled during each shot using SENSE. Therefore, typical image registration can be used to correct inter-shot motion. The method was tested using a simulation of 3D GRASE ASL and an equivalent experiment, where the subject was trained to nod or keep still. The motion correction reduced artefacts, and increased the correlation between cerebral blood flow measurements acquired with and without motion.
Introduction
Arterial Spin Labelling (ASL) is a non-invasive MRI method to measure cerebral blood flow (CBF) [1]. However, ASL is particularly sensitive to motion artefacts, which can cause a significant amount of patient data to be unusable. ASL depends on subtracting two images with a small difference due to inflowing blood, and this difference may easily be overwhelmed by otherwise minor artefacts. Here, a novel retrospective method for correcting motion in multi-shot 3D GRASE is presented and tested, using both simulations and in vivo data.Methods
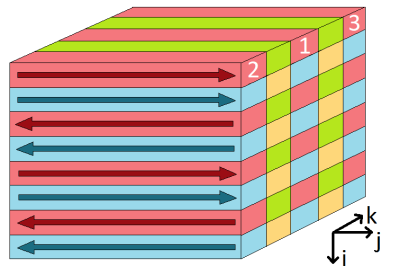
A MATLAB based simulation of the 3D GRASE ASL acquisition sequence was developed, modelling the MRI experiment described below. A BrainWeb [2] simulated brain model was used as input, for realistic structural detail, with grey and white matter CBF set at 60 and 20 ml/100g/min respectively. Realistic typical motion was modelled using resting state fMRI data from a previous study in semantic dementia [3], to which probability distribution functions for rigid motion were fitted. Based on these, a method to retrospectively correct inter-shot motion was developed.For in vivo testing, a healthy volunteer (26 years old) was scanned on a Siemens 3T Prisma. The pulse sequence was multi-shot 3D GRASE PASL with image matrix (62,64,32) and resolution 3.75x3.75x4.5mm. The spin labelling was FAIR Q2TIPS with inversion time (TI1) 2s and Q2TIPS saturation applied after 800ms. The whole k-space was acquired using four interleaved shots (see Figure 1). In addition to the ASL tag and control images, a proton density weighted image was acquired for CBF quantification.
During the scan, the subject was asked to deliberately nod their head continuously within the head coil, during the acquisition of 10 pairs of ASL tag and control images. This movement was timed with the audible quiet period between the spin labelling pulse and signal acquisition, to assure specifically intershot motion occurred. The subject was instructed to remain as still as possible for 10 further ASL pair acquisitions, in order to obtain a motion-free reference data set.
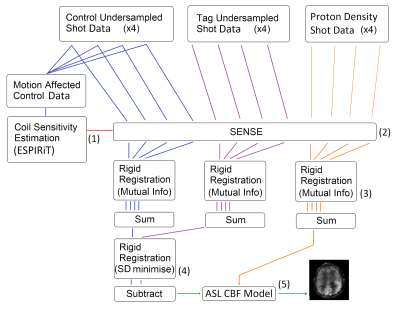
The ESPIRiT algorithm [4] was used to calculate RF coil sensitivity fields from the raw k-space data. ESPIRiT requires as input the complete k-space data in the central region of k-space, which was obtained by combining the k-space points sampled during each of the four shots. The coil sensitivity fields were then used to reconstruct full-field-of-view images from each of the raw undersampled k-space sections acquired in each shot, using SENSE [5]. Then, retrospective correction of intershot motion was performed using standard image registration techniques (see flowchart in Figure 2).
CBF was quantified using the consensus PASL model [1] , and partial volume correction [6] was applied to generate maps of ‘pure’ grey matter (GM) CBF. This required volume fraction maps of GM, white matter and CSF, which were obtained from a 3D T1 MPRAGE image using the NiftySeg [7] segmentation toolbox.
Results
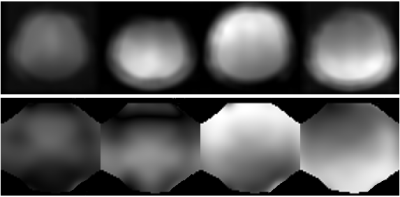
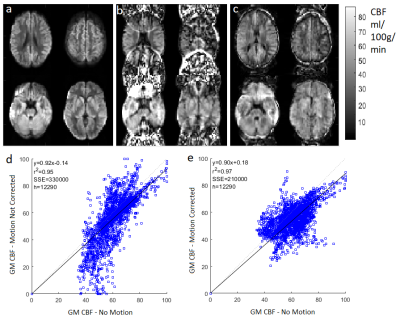
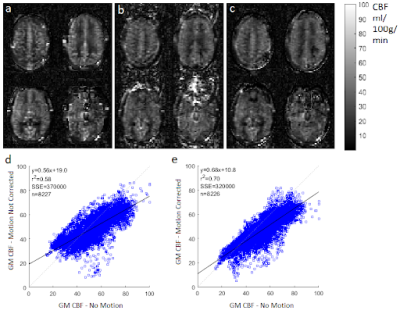
The simulation results demonstrated that ESPIRiT was able to generate sensitivity maps which closely resembled the known input values, even with motion present (Figure 3).The CBF maps produced by the simulation (Figure 4) and the experiment (Figure 5) show significant artefacts due to motion, which were visibly reduced by the ESPIRiT motion correction. The still and motion in vivo data was registered to allow voxel correlation analysis. The correlation graphs show the motion correction improved the CBF correlation between motion affected data and no motion data in both cases, through the lower Sum Square Error (SSE), higher r2, and for the experiment, a line of best fit gradient closer to 1.
In simulation the GM CBF was found to be (50.2 ∓ 6.0) ml/100g/min, significantly underestimating the ground truth value of 60 ml/100g/min. With the ESPIRiT based motion correction, the result was (60.7 ∓ 2.4) ml/100g/min, i.e. consistent with the ground truth value. The no motion in vivo data produced an empirical GM CBF value of (55.2 ∓ 7.0) ml/100g/min, while the deliberate nodding in vivo data yielded (49.6 ∓ 10) which changed to (53.7 ∓ 6.4) after motion correction.
Discussion
The ESPIRiT based motion correction dramatically reduced visible motion artefacts in both simulations and in vivo. In the simulation, the accuracy of GM CBF measurement was significantly improved. In the in vivo data set, the CBF estimate did not change significantly, while in both data sets, the corrected maps had lower structural definition than the no-motion data - which slightly reduced measured CBF resulting in correlation gradients of less than one. However, the motion correction improved the correlation between the data acquired with and without motion according to all associated metrics (Figure 5).As a retrospective motion-correction technique, the proposed method does not require changes to the sequence, such as navigator echoes, nor the additional equipment needed for motion tracking. Data processing and reconstruction took 5 minutes to correct 10 repeats using an Intel i7-3770 core, which is much less computationally intensive than autofocusing [8], an alternative retrospective motion correction technique.
Acknowledgements
UCL Centre for Doctoral Training in Intelligent, Integrated Imaging In Healthcare
PhD position funded by EPSRC
References
[1] Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015 Jan; 73(1): 102–116.
[2] https://brainweb.bic.mni.mcgill.ca/brainweb/
[3] Benhamou, E. Semantic Dementia Study. https://www.nature.com/articles/ s41598-020-72847-1. (2020)
[4] Uecker, Martin and Lai, Peng and Murphy, Mark J and Virtue, Patrick and Elad, Michael and Pauly, John M and Vasanawala, Shreyas S and Lustig, Michael. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magnetic resonance in medicine, 71, 3, 990-1001. (2014)
[5] Pruessmann, Klaas P and Weiger, Markus and Scheidegger, Markus B and Boesiger, Peter. SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine, 42, 5, 952-962. (1999).
[6] Asllani, Iris and Borogovac, Ajna and Brown, Truman R. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magnetic Resonance in Medicine. 60, 6, 1362-1371. (2008)
[7] Cardoso 2015: M. Jorge Cardoso, Marc Modat, Robin Wolz, Andrew Melbourne, David Cash, Daniel Rueckert, and Sebastien Ourselin. Geodesic Information Flows: Spatially-Variant Graphs and Their Application to Segmentation and Fusion. IEEE Transactions on Medical Imaging, 34(9), 1976-1988. (2015)
[8] Atkinson, David, Derek LG Hill, Peter NR Stoyle, Paul E. Summers, Stuart Clare, Richard Bowtell, and Stephen F. Keevil. "Automatic compensation of motion artifacts in MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 41, no. 1 (1999): 163-170.
Figures