2725
Whole-brain Perfusion Mapping at 7T by SAR-efficient Non-segmented 3D EPI-pCASL1Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Center for Neuroscience Imaging Research, Institute of Basic Science, Suwon, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Intelligent Precision Healthcare Convergence, Suwon, Korea, Republic of
Synopsis
Due to high specific absorption rate (SAR), it has not been easy to apply pseudo-continuous arterial spin labeling (pCASL) at 7T human MRI, especially with 3D readouts. In this study, non-segmented 3D-EPI-pCASL is proposed for whole-brain perfusion mapping. SAR was reduced by multiple low-flip-angle water-excitation rectangular RF pulses for 3D EPI. The proposed 3D-EPI-pCASL produced consistent perfusion maps at both 3T and 7T compared to 2D-EPI-pCASL which was available only at 3T because of SAR. The high temporal resolution of the proposed non-segmented 3D-EPI-pCASL enabled us to get a whole-brain 3D pCASL fMRI map at 7T for the first time.
INTRODUCTION
Pseudo-continuous arterial spin labeling (pCASL) has been recommended as one of the non-invasive perfusion imaging techniques.1 While pCASL has been popular at 3T human MRI, pCASL at 7T has not been easy because of its high specific absorption rate (SAR). 7T pCASL has been mostly tried with low SAR 2D readout sequences such as 2D EPI2 and 2D Turbo-FLASH3,4. Although 3D readouts are recommended for ASL in general1, 7T pCASL with 3D readouts has been considered difficult. In this study, a SAR-efficient non-segmented 3D EPI is proposed as a new pCASL readout for whole brain perfusion mapping at both 3T and 7T. SAR is reduced by multiple low-flip-angle water-excitation rectangular RF pulses for 3D EPI. The proposed approach enabled us to map 3D pCASL fMRI in the whole brain at 7T for the first time to our knowledge.METHODS
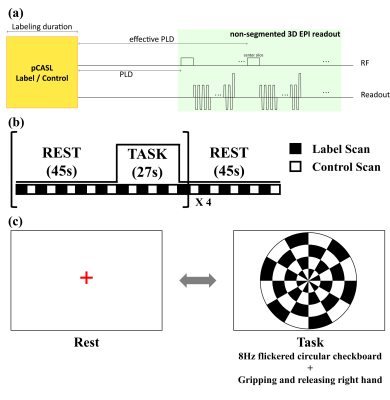
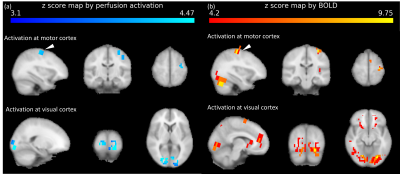
(Readout sequence) The SAR-efficient non-segmented 3D EPI was possible by successive water-excitation rectangular RF pulses with low flip angle (Fig.1.a). The duration of the rectangular RF pulses (4919μs for 3T, 2041μs for 7T) was adjusted to suppress the chemical shift artifacts of fat.5 The absence of additional fat suppression pulses as well as the maximum area for the given RF duration from the rectangular shape enabled us to minimize SAR and shorten imaging time. Successive RF pulses at each partition with low flip angle6, rather than single high-power RF pulse, additionally reduced SAR level of 3D EPI less than 1% at the given TR.(Functional MRI) The preliminary study of whole-brain fMRI was conducted for one subject. To observe accompanying activation of visual and motor cortices, design block of fMRI was determined as shown in Fig. 1b and c.7 Since the proposed sequence is based on gradient echo EPI, BOLD signal could be also detected. Therefore, both perfusion and BOLD activation maps were processed simultaneously using FSL8. (http://www.fmrib.ox.ac.uk/fsl)
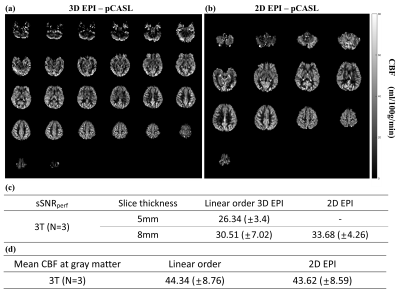
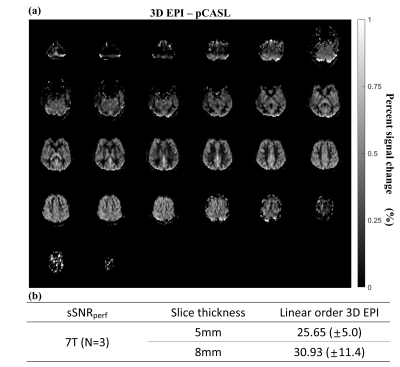
(Data acquisition) All experiments were performed on a 3T MRI (Skyra, Siemens, Erlangen) with a 64-ch head coil and a 7T MRI (Magnetom, Siemens, Erlangen) with 32-ch head coil for three subjects. Labeling parameters were determined as follows: Labeling duration = 1.8s for 3T and 1.5s for 7T, Gslice-selective = 6mT/m, GMean=1mT/m for 3T and 0.6mT/m for 7T, FA=25°, effective PLD=1.5s9. Also, background suppression was applied. The scan parameters were as follows: Base resolution=64, FOV=230mm, slice thickness= 5 or 8mm and 10mm for fMRI, slab thickness=160mm to 200mm (whole brain), FA=12° for 3T and 10° for 7T (Ernst angle), kZ ordering=linear, partial Fourier=6/8 for both phase- and partition- encoding directions, partition TR=38ms for 3T and 31ms for 7T, total TR = 4.25s for 3T and 4.5s for 7T, number of averages = 34 pairs, total scan time = 5.0 -5.5 min. At the 7T MRI, SAR was monitored not to exceed 4W/kg at the brain. As comparison reference, 2D EPI-pCASL was conducted at 3T with following parameters: slice thickness=8mm, gap=2mm, the number of slices=16, FA=90°, partial Fourier=6/8.
(Quantification) Cerebral blood flow (CBF) maps were calculated by the equation at the ASL white paper.1 Also, spatial SNR of a perfusion map (sSNRperf) was computed as the mean signal in gray matter divided by the standard deviation of background noise.10
RESULTS
High-quality perfusion maps were obtained by the proposed 3D EPI-pCASL at both 3T and 7T MRI (Figs. 2 and 3). CBF and sSNRperf values from the proposed 3D EPI-pCASL were consistent with those from the conventional 2D EPI-pCASL, while having more homogeneous post labeling delays and thus more homogeneous perfusion maps across slices. Furthermore, despite usage of a single transmitter/receiver head RF coil at 7T MRI, decent perfusion maps could be acquired without any additional hardware. Moreover, because of high temporal resolution, functional z score maps by perfusion showed the activations at both visual and motor cortices properly. Compared with the BOLD activation maps, perfusion activation maps showed higher local specificity, consistent with the known characteristics.DISCUSSION & CONCLUSION
The proposed non-segmented 3D EPI-pCASL produced qualified perfusion images with SAR-efficiency and high temporal resolution. It was verified by the comparison of CBF quantification and sSNRperf with the conventional 2D EPI-pCASL. With the sufficient quality, the proposed sequence eliminates intrinsic problems of multi-slice 2D and segmented 3D readouts such as heterogeneous post labeling delays across slices or low temporal resolution11. Furthermore, because of high temporal resolution and SAR efficiency, the proposed non-segmented 3D EPI-pCASL could produce the whole brain 3D pCASL-fMRI map at 7T human MRI for the first time to our knowledge. Still, there are potentials to reduce SAR almost by half with no control RF preparation12 and thus increase labeling duration for better perfusion signals and to increase signal to noise ratio by pseudo-centric trajectory.13 The proposed 3D EPI-pCASL can be a good candidate for physiological and functional perfusion mapping at the high fields.Acknowledgements
No acknowledgement found.References
[1] Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine. 2015;73(1):102-116.
[2] Luh WM, Talagala SL, Li TQ, Bandettini PA. Pseudo‐continuous arterial spin labeling at 7 T for human brain: estimation and correction for off‐resonance effects using a Prescan. Magnetic Resonance in Medicine. 2013;69(2):402-410.
[3] Zuo Z, Wang R, Zhuo Y, Xue R, Lawrence KSS, Wang DJ. Turbo-FLASH based arterial spin labeled perfusion MRI at 7 T. PloS one. 2013;8(6):e66612.
[4] Wang Y, Moeller S, Li X, et al. Simultaneous multi-slice Turbo-FLASH imaging with CAIPIRINHA for whole brain distortion-free pseudo-continuous arterial spin labeling at 3 and 7 T. Neuroimage. 2015;113:279-288.
[5] Stirnberg R, Brenner D, Stöcker T, Shah NJ. Rapid fat suppression for three‐dimensional echo planar imaging with minimized specific absorption rate. Magnetic resonance in medicine. 2016;76(5):1517-1523.
[6] Gai ND, Chou YY, Pham D, Butman JA. Reduced distortion artifact whole brain CBF mapping using blip-reversed non-segmented 3D echo planar imaging with pseudo-continuous arterial spin labeling. Magnetic resonance imaging. 2017;44:119-124.
[7] Fernández‐Seara MA, Wang Z, Wang J, et al. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005;54(5):1241-1247.
[8] Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790.
[9] Gai ND, Butman JA. Determining the optimal postlabeling delay for arterial spin labeling using subject‐specific estimates of blood velocity in the carotid artery. Journal of Magnetic Resonance Imaging. 2019;50(3):951-960.
[10] Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo‐continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O‐water PET in elderly subjects at risk for Alzheimer's disease. NMR in Biomedicine. 2010;23(3):286-293.
[11] Shao X, Tisdall MD, Wang DJ, van der Kouwe AJW. Prospective motion correction for 3D GRASE pCASL with volumetric navigators. Paper presented at: Proceedings of the International Society for Magnetic Resonance in Medicine... Scientific Meeting and Exhibition. International Society for Magnetic Resonance in Medicine. Scientific Meeting and Exhibition2017.
[12] Han PK, Choi SH, Park SH. Investigation of control scans in pseudo‐continuous arterial spin labeling (p CASL): Strategies for improving sensitivity and reliability of p CASL. Magnetic resonance in medicine. 2017;78(3):917-929.
[13] Lee HS, Hwang SH, Park J, Park SH. Single-shot pseudo-centric EPI for Magnetization-Prepared Imaging. 2020 ISMRM & SMRT VIRTUAL CONFERENCE & EXHIBITION. No. Program# 3735. 2020.
Figures