2724
Gradient adjustments for improved pcASL exploiting a B1+ shimmed labeling train1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 3Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Institute of Neuro-Radiology, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany
Synopsis
Pseudo-continuous arterial spin labeling (pcASL) at 7T suffers from insufficient B1+-amplitudes and specific absorption rate (SAR) constraints. Even with B1+ phase-only or phase/amplitude shimming for the labeling the resulting inversion is suboptimal. In this work, we performed Bloch simulations to examine the impact of thicker pcASL labeling slices by adjusting the slice-selective gradient of a B1+-shimmed labeling train exploiting Variable-Rate Selective Excitation. The findings were evaluated experimentally in 5 healthy volunteers. Using lower slice-selective gradients and subsequently longer exposure of the spins to the pcASL labeling train, the perfusion images improved according to gray matter temporal signal-to-noise ratio by 35%.
Introduction:
Arterial spin labeling (ASL) in ultra-high field MRI benefits from prolonged T1-times and higher signal-to-noise ratio.1 However, at 7 Tesla, the recommended method for ASL2, pseudo-continuous arterial spin labeling (pcASL), suffers from specific absorption rate (SAR) constraints and insufficient B1+ amplitudes for labeling. To reduce SAR, Variable-Rate Selective Excitation (VERSE) pulses promise great advantage for the labeling/control scheme3,4. To overcome B1+ inhomogeneity and B1+ inefficiency for labeling pulses, B1+ phase-only shimming5 as well as phase/amplitude shimming was introduced4. However, this still leads to suboptimal inversion amplitudes4. Thicker labeling slices and subsequently lower maximum gradients could improve labeling efficiency, even under suboptimal B1+ constraints, due to longer exposure of the spins to the pcASL labeling train. In this work, we simulated the impact of different slice-selective gradients of the pcASL labeling train by Bloch simulations and validated the results experimentally.Methods:
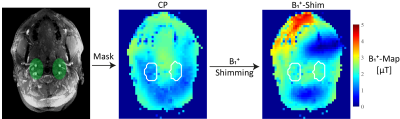
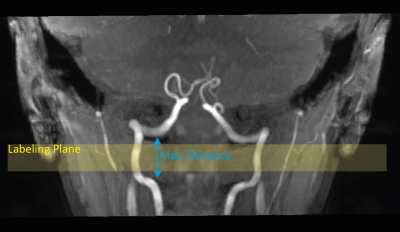
Bloch simulations were performed for the below described labeling train using different B1+ amplitudes (0.8:0.1:3.6µT), different slice-selective gradients strengths (Gmax=2.8:0.7:7.0mT/m) and a mean velocity of 40cm/s. The average gradient strength was kept constant to a proposed ratio6 of $$$\frac{G_{ave}}{G_{max}}=\frac{1}{7}$$$ . The simulation results were validated on a 7T MR system (MAGNETOM Terra, Siemens Healthcare GmbH, Erlangen, Germany) using a 32-channel Rx/8Tx head-coil (Nova Medical, Wilmington, Massachusetts, USA) and carried out in accordance with the institutional guidelines and with approval of the local ethics committee (Friedrich-Alexander University (FAU) Erlangen-Nürnberg, Germany). An unbalanced pCASL labeling scheme (TPulse=500μs, TGap=1200μs) was modified by using VERSE pulses7 (50% amplitude reduction, GVERSE,max = 10mT/m). Further, a hybrid B1+ phase shim was applied that trades B1+ homogeneity and transmit efficiency, based on acquired relative B1+ maps8. To visualize the main feeding arteries in the V3 segment, TOF images were acquired (compare Figure 1, Figure 2). For the hybrid B1+ phase shim, the cost function $$$cost=\left(1-\lambda\right)\cdot CoV^{2} + \lambda\cdot\eta^{2}$$$ was minimized (weight λ=0.3), with coefficient of variation $$$ CoV=\frac{std\left(\left|\sum_{c=1}^C B_{1,c}^{+}(r)\right|\right)}{mean\left(\left|\sum_{c=1}^C B_{1,c}^{+}(r)\right|\right)} $$$ and effiency $$$ \eta = mean\left(\frac{\left|\sum_{c=1}^C B_{1,c}^{+}(r)\right|}{\sum_{c=1}^C \left|B_{1,c}^{+}(r)\right|}\right) $$$ For the readout a 2D echo planar imaging (EPI) sequence was used with following parameters: resolution=2×2×4mm3, 15 slices, PAT=2, TE=15ms, flip angle=90°, TR=6600-8500ms (depending on SAR). In total 5 subjects (mean age: 25.6±2.1years) were measured with the optimized slice-selective gradient, according to the Bloch simulation and compared to a thin labeling slice (high slice-selective gradient) as suggested by Alsop et al.2. The gradient amplitude was restricted by VERSE and set to 7mT/m. Additional, a magnetization prepared rapid gradient echo9 was acquired to segment gray matter, white matter and cerebrospinal fluid. The pcASL images underwent postprocessing using SPM12 (Wellcome Trust Centre for Neuroimaging)10 as well as distortion correction11. All pcASL measurements were performed twice (each 37 repetitions) and the mean tSNR in gray matter was calculated for evaluation.Results:
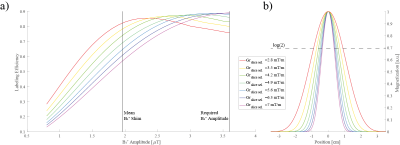
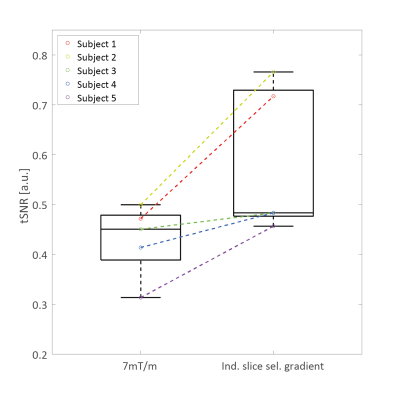
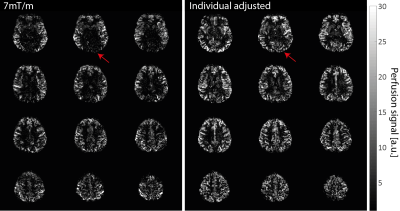
The simulation of suboptimal B1+ amplitudes shows, that lower slice-selective gradients lead to a higher labeling efficiency (Figure 3a). However, with lower slice-selective gradients the labeling slice thickness increases (Figure 3b). Since at pcASL, the blood should pass through the labeling slice perpendicularly - and the labeling was applied in the V3 segment - it is restricted by the distance between the bends of the vessels (Figure 2). Therefore, it was adjusted individually for the measured subjects (slice thickness limit was set to the full width at log(2) of the normalized magnetization). Figure 4 shows the tSNR of both measurements for all subjects including the individual slice-selective gradient. The mean tSNR over all subjects for the 7mT/m slice-selective gradient was 0.43±0.06 and for the individual adjusted gradient 0.58±0.13, resulting in an average tSNR gain of 35%. An examplary result of the measurements is given in Figure 5. The red arrow marks higher perfusion signal in the individual adjusted sequence compared to 7mT/m.Discussion and Conclusion:
The labeling efficiency and therefore the tSNR of the measurements was improved by individual gradient adjustments as addition to B1+ shimming. Nevertheless, due to the restriction of the labeling thickness, because of individual anatomical structure, it was not possible to acquire the optimized slice-selective gradient, according to the Bloch simulation. However, positioning the labeling below the V3 segment is not feasible due to the restricted coil coverage.Acknowledgements
No acknowledgement found.References
1. Teeuwisse, W. M., Webb, A. & Osch, J. P. Arterial Spin Labeling at Ultra-High Field: All That Glittersis Not Gold. IMA 20, 62-70 (2010).
2. Alsop, D. C. et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73, 102-116, doi:10.1002/mrm.25197 (2015).
3. Boland M, Stirnberg R, Pracht ED, Stöcker T. Robust and SAR-efficient whole-brain pseudo-continuous ASL at 7T. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019.
4. Tong, Y., Jezzard, P., Okell, T. W. & Clarke, W. T. Improving PCASL at ultra-high field using a VERSE-guided parallel transmission strategy. Magn Reson Med, doi:10.1002/mrm.28173 (2020).
5. Li X, W. D., Wu
X, Van de Moortele PF, Ugurbil K, Metzger GJ. In Proceedings of the 26th
Annual Meeting of ISMRM,Paris, France, 2018.
6. Zhao, L., Vidorreta, M., Soman, S., Detre, J. A. & Alsop, D. C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn Reson Med 78, 1342-1351, doi:10.1002/mrm.26513 (2017).
7. Schmitter, S. et al. Contrast enhancement in TOF cerebral angiography at 7 T using saturation and MT pulses under SAR constraints: impact of VERSE and sparse pulses. Magn Reson Med 68, 188-197, doi:10.1002/mrm.23226 (2012).
8. Fautz HP, Vogel M, Gross P, Kerr A, Zhu Y. B1 mapping of coil arrays for parallel transmission. In Proceedings of the 22nd Annual Meeting of ISMRM, Toronto, Canada, 2008.
9. Mugler, J. P., 3rd & Brookeman, J. R. Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging 1, 561-567, doi:10.1002/jmri.1880010509 (1991).
10. Wang, Z. et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic resonance imaging 26, 261-269, doi:10.1016/j.mri.2007.07.003 (2008).
11. Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870-888, doi:10.1016/S1053-8119(03)00336-7 (2003).
Figures