2712
The neuro-brain functional mechanisms that cause different efficacy of transcutaneous auricular vagus nerve stimulation on primary insomnia1Department of chinese medicine, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China, 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China, 3Philips Healthcare, Shanghai, China
Synopsis
Recently, some studies have reported the efficacy of transcutaneous auricular vagus nerve stimulation (taVNS) on primary insomnia. However, its effective rate fluctuated greatly. Therefore, our study aimed at studying the reasons that caused the different efficacy of taVNS.
Short abstract
Our study aimed at comparing the differences of fractional amplitude of low-frequency fluctuation (fALFF) value and heart rate variability (HRV) between “effective group (group A)” and “non-effective group (group B)” during continuous taVNS to analyze the neuro-brain functional mechanisms that caused the different efficacy of taVNS to primary insomnia (PI), so as to provide clinical and theoretical basis for individualized treatment of taVNS. We got these conclusions that the regulation of SMN to HRV during continuous taVNS are may the mechanism that caused the different efficacy of taVNS. The HRV indicators and fALFF value of SMN during the continuous taVNS were might the bio-markers that could predict the efficacy of taVNS on insomnia.Abstract
Introduction: Recently, some studies have reported the efficacy of transcutaneous auricular vagus nerve stimulation (taVNS) on primary insomnia. However, its effective rate fluctuated greatly. Therefore, exploring the reasons that caused the different efficacy of taVNS were the key to practice individual treatment in clinic. Our study aimed at comparing the differences of fractional amplitude of low-frequency fluctuation (fALFF) value derived from functional magnetic resonance imaging (fMRI) analysis and heart rate variability (HRV) derived from electrocardiography (ECG) between “effective group (group A)” and “non-effective group (group B)” during continuous taVNS to analyze the neuro-brain functional mechanisms that caused the different efficacy, so as to provide clinical and theoretical basis for individualized treatment of taVNS.Materials and Methods:20 effective and 20 ineffective Insomnia patients that had been treated by taVNS (Fig.1 and 2) were recruited from the Department of Sleep Disorder, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, and were treated by taVNS for 4 weeks. The evaluetion of efficacy criteria were adopted according to the score-reduction rate of PSQI, when it ≥25% was defined as effective and regarded as group A, when it <25% was defined as ineffective and regarded as group B. Then using fMRI and ECG gating to record the fALFF and HRV data before and during continuous taVNS between the two groups. Then, analyzing the correlation among efficacy (score-reduction rate of PSQI or differences of PSQI before and after treatment) fALFF value, and HRV value during continuous taVNS
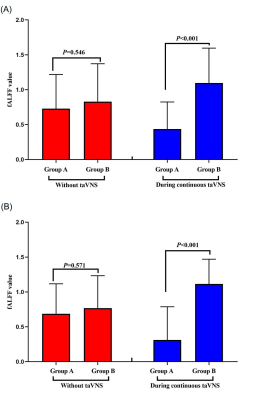
Results:The value of HRV indicators (rMSSD, PNN50 and HF) in group A were higher than group B during the continuous taVNS (Fig. 3). The fALFF value of right cerebellum, right medial superior frontal gyrus (mSFG), and bilateral supplementary motor area (SMA) of group A, which belong to sensorimotor network (SMN) was significant lower than group B during continuous taVNS and there was no significant difference of these brain regions when without taVNS (Fig. 3 and 4).
Conclusions:The vagus nerve of patients who have different efficacy after treating by taVNS may have different sensitivities to taVNS. The regulation of SMN to HRV during continuous taVNS are may the mechanism that caused the different efficacy of taVNS. The HRV indicators and fALFF value of SMN during the continuous taVNS were might the bio-markers that could predict the efficacy of taVNS on insomnia.
Acknowledgements
The authors would like to thank all volunteers who took part in the study. The authors thank Dr. Tian-jing Zhang for their experiment assistance and guidance in data analysis of HRV.References
1. Zhao B, Bi Y, Li L, et al. The Instant Spontaneous Neuronal Activity Modulation of Transcutaneous Auricular Vagus Nerve Stimulation on Patients With Primary Insomnia[J]. Front Neurosci, 2020, 14: 205.
2. Butt MF, Albusoda A, Farmer AD, et al. The anatomical basis for transcutaneous auricular vagus nerve stimulation[J]. J Anat, 2020, 236(4): 588-611.
3. Jiao Y, Guo X, Luo M, et al. Effect of Transcutaneous Vagus Nerve Stimulation at Auricular Concha for Insomnia: A Randomized Clinical Trial[J]. Evid Based Complement Alternat Med, 2020, 2020: 6049891.
4. Englot DJ, Rolston JD, Wright CW, et al. Rates and Predictors of Seizure Freedom With Vagus Nerve Stimulation for Intractable Epilepsy[J]. Neurosurgery, 2016, 79(3): 345-353.
5. Ojeda D, Le Rolle V, Romero-Ugalde HM, et al. Sensitivity Analysis of Vagus Nerve Stimulation Parameters on Acute Cardiac Autonomic Responses: Chronotropic, Inotropic and Dromotropic Effects[J]. PLoS One, 2016, 11(9): e163734.
[6] Liu CH, Liu CZ, Zhang J, et al. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms[J]. Brain Res, 2016, 1648(Pt A): 317-324.
Figures
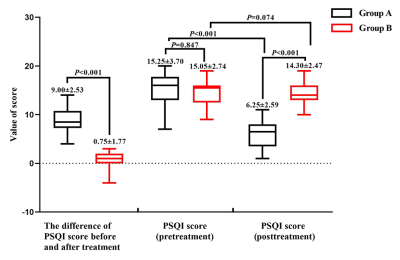
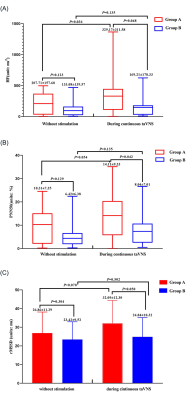
Figure 3: The HRV indicators of group A and B without and during the continuous taVNS. (A): The value of HF of group A and B without and during continuous taVNS. (B): The value of PNN50 of group A and B without and during continuous taVNS. (C): The value of rMSSD of group A and B without and during the continuous taVNS.
Note: HRV, heart rate viability; rMSSD, root mean square of successive differences; ms, millisecond; taVNS, transcutaneous auricular vagus nerve stimulation; PNN50, percetange of adjacent NN intervals differing by more than 50 milliseconds; HF, high frequency.
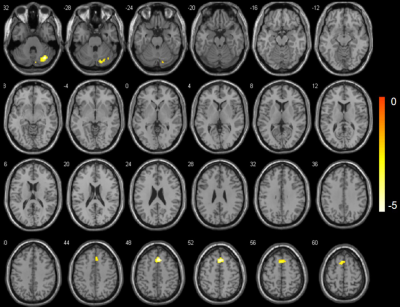
Figure 4: The changes of fALFF value between group A and B during continuous taVNS. The cold color indicates the regions showing decreased fALFF value during continuous taVNS.
The neuronal activity during continuous taVNS showed the fALFF value of right cerebellum, right medial superior frontal gyrus (mSFG), and bilateral supplementary motor area (SMA) of group A were lower tha group B.