2704
Initial clinical evaluation of locally low-rank denoising on motor areas for task-based presurgical functional MRI1Mayo Clinic Graduate School of Biomedical Sciences, Rochester, MN, United States, 2Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
In a preliminary evaluation of task-based motor fMRI data of five healthy subjects, locally low-rank (LLR) denoising is evaluated for clinical performance. Complex-valued timecourse echo planar imaging (EPI) data from finger-tapping exams were LLR-denoised and equivalently processed alongside control images. Statistical thresholds of LLR-denoised and control data were assessed by four board-certified neuroradiologists for four specific clinically relevant clusters and overall. LLR denoising predominantly yields increased thresholds for four specific cluster regions and aggregate maps in a preliminary analysis.
Introduction
Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI)1 enables noninvasive mapping of eloquent brain tissue.2,3 fMRI has increasingly gained importance for presurgical and radiotherapy planning, with improved therapeutic outcomes for brain tumor patients.4 fMRI is inherently limited by fast scanning acquisition strategies used in data acquisition, including relatively low signal-to-noise ratio and imaging artifacts. These limitations contribute to uncertainties within derived fMRI activation maps, confounding clinical interpretation and hindering widespread clinical implementation of resting-state fMRI.5,6We have adapted locally low-rank (LLR)7-10 regularization to complex-valued echo planar imaging (EPI) time series data for use in task-based fMRI. Data processed with LLR denoising resulted in fMRI activation maps with increased statistical significance and additional activation areas compared to identically processed baseline data.11 Here we report a preliminary clinical evaluation by board-certified neuroradiologists assessing fMRI activation from a bilateral hand-motion task.
Methods
Define $$$G=X+Z$$$ as a set of $$$T$$$ $$$N\times N$$$ complex-valued MR images rearranged as a spatiotemporally concatenated $$$N^2\times T$$$ Casorati matrix, with target signal $$$X$$$ and $$$Z\sim CN(0,\sigma^2)$$$ denoting zero-mean complex Gaussian noise. Then, briefly, LLR denoising comprises singular value thresholding12 implemented blockwise:$$\hat{X}=C^{-1}\sum_{b\in\Omega}R_b^*\big\{SVT_{\lambda/2}\{R_b G\}\big\}$$
denoting $$$R_bG$$$ as a binary operator $$$R_b$$$ selecting from $$$G$$$ a block $$$b$$$ of size $$$\beta$$$ from the set of overlapping blocks $$$\Omega$$$, with $$$C=\sum_{b\in\Omega}R_b^*{R_b}$$$ and threshold parameter $$$\lambda$$$.
Five healthy volunteers were scanned on a compact 3T MRI scanner13 under an IRB-approved protocol. Imaging parameters were $$$N_x\times N_y\times N_z\times N_t = 128\times 128\times 75\times 120$$$ at a $$$24$$$cm FOV ($$$1.9$$$mm isotropic resolution), with $$$TR/TE=2000/30$$$ms and a $$$77^\circ$$$ flip angle. Simultaneous multi-slice acceleration14-18 $$$(R=3)$$$ was used to achieve whole-brain acquisition at these slice-selective spatial and temporal resolutions. In-plane acceleration was not used. A sagittal $$$1.0$$$mm isotropic MPRAGE anatomic scan enabled registration. Subjects completed an alternating bilateral finger tapping functional task.
Image reconstruction was performed in C++ yielding complex-valued timecourse image data. LLR denoising was completed with $$$\beta=8,\lambda=5$$$ before saving magnitude image data. $$$8$$$s of initial TRs were discarded prior to LLR denoising or functional processing depending on processing variant, to suppress transient signal.19 Scanner DICOM (control) data and LLR-denoised data were processed equivalently using Prism Process,20 which utilizes Analysis of Functional Neuroimages (AFNI)21 functionality in processing. Spatial smoothing of timecourse EPI data via Gaussian blur (AFNI: 3dmerge) and $$$t$$$-statistical data via finite difference diffusion equation approximation (AFNI: 3dBlurtoFWHM) was completed in functional processing, following local clinical pipeline preferences.
Four board-certified neuroradiologists experienced in clinical task-based fMRI thresholded non-dominant and dominant primary motor cortex clusters, the supplementary motor area (SMA), and cerebellum, by consensus in real time, emulating routine clinical practice at our institution while assessing additional specific cluster regions for methodological analysis. Activation maps were thresholded using Prism View,20 the FDA-approved fMRI visualization interface used clinically at our institution. Following thresholding of specific clusters, maps were thresholded in aggregate as if reading clinically. Consensus thresholds for each cluster and aggregate maps were recorded.
Results
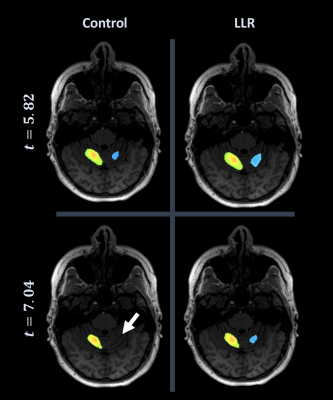
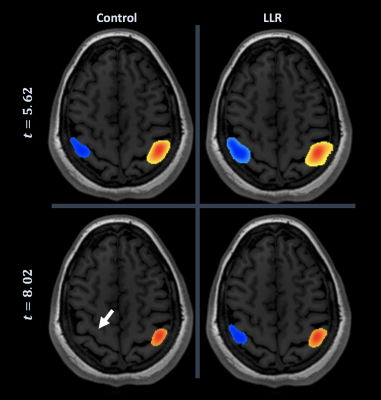
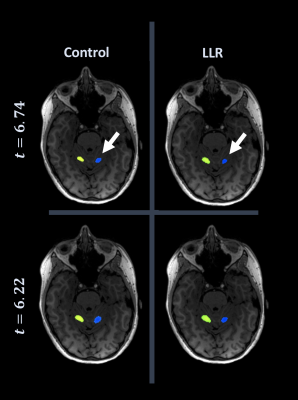
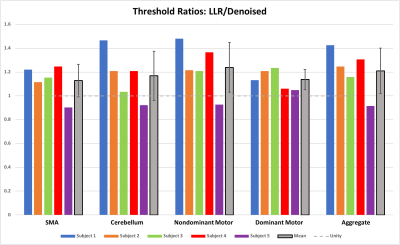
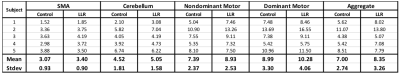
Figures 1 through 3 show distinct individual subject results. Figure 4 shows ratios of LLR-denoised to control thresholds for specific cluster region thresholds. Figure 5 tabulates all thresholds recorded in consensus. Figures 1 and 2 illustrate examples of LLR-denoised data attaining higher statistical thresholds when thresholded by readers, with loss of bilateral activation for baseline data at thresholds set for LLR-denoised data. Figure 3 illustrates behavior of the sole case for which consensus threshold of LLR data was exceeded by control, but without bilateral activation loss as exhibited by control at higher thresholds in other subjects. Threshold ratios tended to substantially exceed $$$1$$$ for all sets of clusters for all but one subject. Mean and standard deviation of LLR/control threshold ratios for the SMA, cerebellum, nondominant motor, dominant motor, and aggregate maps were $$$1.13\pm{0.14},1.17\pm{0.21},1.24\pm{0.21},1.14\pm{0.08},$$$ and $$$1.21\pm{0.19}$$$ respectively.Discussion
LLR denoising was shown in this preliminary evaluation to allow higher statistical thresholds of fMRI activation maps. Compared to conventionally processed fMRI maps, areas of activity on LLR-denoised results were larger in volume, and tended to reveal additional activation areas and/or volumes of existing clusters, enabling more aggressive statistical thresholding for improved confidence in localizing eloquent tissue. More subjects and task paradigm variants, with corresponding statistical analysis, are required to systematically demonstrate the clinical utility of this technique and will be performed when feasible.LLR denoising is straightforward in tuning adjustable parameters and is easily parallelizable, with reasonable processing times depending on block size. It is applicable to any fMRI dataset with an available complex-valued reconstruction, not requiring changes in acquisition technique or scan time, and increases diagnostic utility and clinical confidence of derived fMRI activation maps.
Conclusion
Functional MRI activation maps for a bilateral hand motor task obtained in five subjects processed without and with locally low rank (LLR) denoising were evaluated by four board-certified neuroradiologists. Compared to routine fMRI post-processing, LLR denoising resulted in functional maps with higher statistical thresholds of fMRI activation in four specific functional cluster regions and overall in a preliminary evaluation. LLR denoising enables visualization of activity which could be suppressed by standard analysis, or more strongly thresholded for increased statistical confidence in active tissue localization.Acknowledgements
This work was supported by NIH Grants U01EB024450 and U01 EB026979 and the National Science Foundation (NSF) Graduate Research Fellowship Program (GRFP).References
1. Ogawa S, Lee TM et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990; 87(24):9868-9872.
2. Black DF, Vachha B et al. American Society of Functional Neuroradiology–Recommended fMRI Paradigm Algorithms for Presurgical Language Assessment. Am J Neuroradiol. 2017, 38:E65–E73.
3. Mahdavi A, Azar R et al. "Functional MRI in clinical practice: Assessment of language and motor for pre-surgical planning". Neuroradiol J. 2015; 28(5):468-473.
4. Vysotski S, Madura C et al. Preoperative FMRI Associated with Decreased Mortality and Morbidity in Brain Tumor Patients. Interdiscip Neurosurg. 2018; 13:40-45.
5. O'Connor EE, Zeffiro TA. Why is Clinical fMRI in a Resting State? Front Neurol. 2019; 10:420.
6. Specht K. Current Challenges in Translational and Clinical fMRI and Future Directions. Front Psychiatry. 2019; 10:924.
7. Trzasko JD, Manduca A. Local versus Global Low-Rank Promotion in Dynamic MRI Series Reconstruction. Proc Intl Soc Mag Reson Med. 2011; 4371.
8. Candes EJ; Sing-Long CA; Trzasko JD. Unbiased Risk Estimates for Singular Value Thresholding and Spectral Estimators. IEEE Trans Signal Process. 2013; 61(19):4643-4657.
9. Zhang T, Pauly JM, Levesque IR. Accelerating Parameter Mapping with a Locally Low Rank Constraint. Magn Reson Med. 2015; 73(2):655-661.
10. Hu Y, Levine EG et al. Motion‐robust reconstruction of multishot diffusion‐weighted images without phase estimation through locally low‐rank regularization. Magn Reson Med. 2019; 81(2):1181-1190.
11. Meyer NK, Campeau NG et al. Locally low-rank denoising of complex-valued EPI reconstructions preceding task fMRI analysis. Proc Intl Soc Mag Reson Med. 2020; 3877.
12. Cai JF, Candes EJ, Shen Z. A Singular Value Thresholding Algorithm for Matrix Completion. SIAM J Optim. 2010; 20(4):1956-1982.
13. Foo TK, Laskaris E et al. Lightweight, compact, and high-performance 3T MR system for imaging the brain and extremities. Magn Reson Med. 2018;80:2232-2245.
14. Larkman DJ, Hajnal JV et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001; 13(2):313-317.
15. Moeller S, Yacoub E et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010; 63(5):1144-1153.
16. Setsompop K, Gagoski BA et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012; 67(5):1210-1224.
17. Barth M, Breuer F et al. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med. 2016; 75(1):63-81.
18. Mark IT, Black DF et al. Higher temporal resolution multiband fMRI provides improved presurgical language maps. Neuroradiology 2020.
19. Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. NeuroImage 2017; 154:128-149.
20. Prism Clinical Imaging, RRID:SCR_016977; Elm Grove, WI.
21. Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996; 29:162-173.
Figures