2681
Functional imaging of the anterior temporal lobe: effects of acceleration, multi-echo acquisition, and reconstruction methods
Matteo Visconti di Oleggio Castello1, Valentina Borghesani2, Katherine P. Rankin3, Jack L. Gallant1, and An T. Vu3,4
1University of California, Berkeley, Berkeley, CA, United States, 2Université de Montréal, Montréal, QC, Canada, 3University of California, San Francisco, San Francisco, CA, United States, 4San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States
1University of California, Berkeley, Berkeley, CA, United States, 2Université de Montréal, Montréal, QC, Canada, 3University of California, San Francisco, San Francisco, CA, United States, 4San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States
Synopsis
The anterior temporal lobe (ATL) is an important brain structure that is hard to functionally image with EPI sequences due to signal dropout and low SNR. To address this problem, we developed a whole-brain EPI sequence with increased SNR in the ATL. We evaluated single- and multi-echo sequences while varying in-plane and multiband acceleration with different reconstruction methods. Sequence evaluation was based on tSNR and functional metrics from computational neuroscience (explainable variance and encoding model performance). The highest functional SNR in ATL and across the cortex was provided by multi-echo sequences with moderate iPAT and MB acceleration, and LeakBlock reconstruction.
Introduction
The anterior temporal lobe (ATL) is an important brain structure that is targeted by frontotemporal dementia, one of the most common neurodegenerative disorders in individuals under the age of 651. Because conventional whole-brain EPI sequences have high signal dropout and low SNR in ATL, most clinical and neuroscience fMRI studies do not sample sufficient signal to functionally characterize this area. To address this problem, we developed an EPI sequence with increased SNR in the ATL and in the whole cortex.Methods
We tested seven sequences based on the CMRR multiband sequence2–4 on a 3T Siemens Prisma with a 64ch head-coil (Fig. 1). The amount of in-plane (iPAT1-2) and multiband acceleration (MB2-3), and the number of echoes (ME1,3) were varied systematically. FLEET5 reference scan mode was used for iPAT. MB RF phase scrambling was used for MB3. Excitation RF pulse duration was shortened to 2ms to minimize dropout due to through-plane dephasing. Sequences were acquired with LeakBlock6 on and SENSE1 off, and retro-reconstructed without LeakBlock and/or with POCS7. All sequences had a 2s TR, 2.6mm isotropic voxels, and ⅞ Partial Fourier.Because high TSNR8 alone does not guarantee high SNR for functional analyses, sequences were also evaluated with model-based metrics from computational neuroscience, such as explainable variance (EV) and encoding model performance (MP)9,10. One healthy participant (34 yo, male) performed a short resting-state task (50 volumes) to quantify TSNR, and a movie-watching task (466 volumes) to quantify functional SNR (FSNR). In the movie condition the same movie was repeated four times for each tested sequence.
TSNR and FSNR (EV and MP) quantify complementary properties that an optimal functional sequence should maximize. TSNR quantifies signal stability, and it was computed as the temporal mean divided by the temporal standard deviation. EV quantifies the noise ceiling for an encoding model, and it was computed as the mean R2 score between brain responses to each repetition of the movie and the average response across repetitions. MP quantifies how well a sequence preserves the functional characterization recovered by an existing encoding model11–13. The encoding model was fit on previously collected data from the same participant and it was used to predict brain activity in response to the movie. MP was quantified as the correlation between model prediction and brain data. Whole-brain results in Figs. 4A,5A show EV and MP for each sequence in the same top 20,000 best-predicted voxels in an independent dataset.
Functional data was minimally preprocessed. Each run was motion-corrected, distortion-corrected, and aligned to the participant’s anatomical volume. For multi-echo sequences, transformations were estimated from the first echo and applied to all echoes. Volumes from separate echoes were recombined as in Posse et al14. To reduce confounds when comparing sequences, data was only linearly detrended in time. Spatial smoothing and slice-timing correction were not performed.
Results
For single-echo sequences, increased iPAT and MB acceleration reduced TSNR, except for IPAT1 (Fig. 2A). Multi-echo sequences outperformed single-echo sequences in ATL. IPAT2 ME3 MB2 with performance mode was the best in both the ATL and the whole cortex (Fig. 2B). Without performance mode, MB2 and MB3 with LeakBlock performed similarly. However, MB3 might outperform MB2 with a shorter and optimized TR. For all sequences, LeakBlock improved TSNR and reduced signal leakage outside the brain (Fig. 3). POCS reconstruction reduced TSNR, as expected due to reduced PSF blurring.The IPAT2 ME3 MB2 sequence performed best also according to FSNR. While both IPAT1 ME1 and IPAT2 ME3 showed high explainable variance in the ATL and the whole cortex, IPAT2 ME3 was the best in ATL (Fig. 4). The ME3 sequence showed the highest model performance in the whole cortex, and it performed similarly to IPAT2 ME1 in the ATL (Fig. 5A). However, model performance across the temporal lobe was much higher for ME3 than the other sequences (Fig. 5B). Consistent with the TSNR results, LeakBlock improved FSNR, especially with iPAT (Figs. 4A,5A). POCS reconstruction provided a modest boost for explainable variance in ATL (Fig. 4A).
Discussion
The IPAT2 ME3 MB2 sequence was the best in both the ATL and the whole cortex. This sequence was validated with TSNR and with functional metrics such as explainable variance and model performance. These metrics test functional SNR directly, and they are commonly used in neuroscience experiments to infer functional properties of the brain. This provides confidence that the IPAT2 ME3 MB2 sequence will perform well in future neuroscience experiments.To acquire multiple echoes, acceleration must be increased to allow a short first echo. While increased acceleration alone can reduce SNR, sampling the BOLD response at different TEs can increase both TSNR and FSNR. Finally, while LeakBlock improved all IPAT2 sequences, we tested only low MB accelerations. This improvement might not generalize to higher acceleration factors.
Conclusion
Optimized EPI sequences are fundamental to study neurodegenerative disorders that target areas with signal dropout and low SNR. Here we developed a whole-brain, multi-echo EPI sequence with increased functional SNR in ATL and in the whole cortex. This sequence can benefit any clinical and basic research study that uses fMRI to characterize the functional properties of brain areas in healthy participants and patients.Acknowledgements
This project was supported by an NIH/NIA grant RF1 AG029577 to Katherine P. Rankin and a UCSF REAC award to An T. Vu.References
- Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386(10004):1672-1682.2.
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224.3.
- Xu J, Moeller S, Auerbach EJ, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 2013;83:991-1001.4.
- Moeller S, Yacoub E, Olman CA, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144-1153.5.
- Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016;75(2):665-679.6.
- Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med. 2014;72(1):93-102.7.
- McGibney G, Smith MR, Nichols ST, Crawley A. Quantitative evaluation of several partial Fourier reconstruction algorithms used in MRI. Magn Reson Med. 1993;30(1):51-59.8.
- Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. Neuroimage. 2007;34(2):565-574.9.
- Schoppe O, Harper NS, Willmore BDB, King AJ, Schnupp JWH. Measuring the Performance of Neural Models. Front Comput Neurosci. 2016;10:10.10.
- Hsu A, Borst A, Theunissen FE. Quantifying variability in neural responses and its application for the validation of model predictions. Network. 2004;15(2):91-109.11.
- Wu MC-K, David SV, Gallant JL. Complete functional characterization of sensory neurons by system identification. Annu Rev Neurosci. 2006;29:477-505.12.
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, Gallant JL. Natural speech reveals the semantic maps that tile human cerebral cortex. Nature. 2016;532(7600):453-458.13.
- Naselaris T, Kay KN, Nishimoto S, Gallant JL. Encoding and decoding in fMRI. Neuroimage. 2011;56(2):400-410.14.
- Posse S, Wiese S, Gembris D, et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 1999;42(1):87-97.
Figures

Figure 1. Sequences tested. Each row indicates the metric used to assess the sequence (temporal SNR, functional SNR), in-plane acceleration factor (iPAT), multiband acceleration factor (MB), partial fourier factor (PF), TR, and echo times (TE1-3). Sequences were collected with LeakBlock on (LB ON), and were retro-reconstructed with either LeakBlock off (LB OFF), LeakBlock on and POCS (LB ON + POCS), or POCS only (LB OFF + POCS). PERFGR: gradients in performance mode.
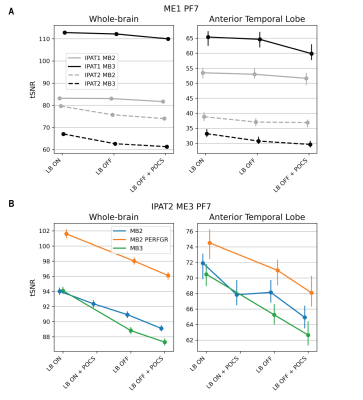
Figure 2. Median TSNR (y-axis) for each reconstruction method (x-axis) for the tested sequences in a cortical (left) and ATL mask (right). A) Single-echo sequences. Combined IPAT and MB acceleration reduce TSNR. B) Multi-echo sequences. MB2 with gradients in performance mode shows the highest TSNR in ATL. Multi-echo sequences have higher TSNR in ATL than single-echo sequences. For all sequences, LB ON outperforms other reconstruction methods.
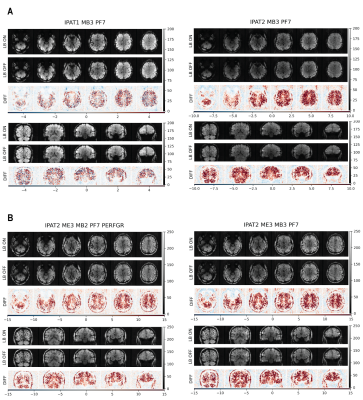
Figure 3. Axial and coronal slices showing TSNR for the best-performing (left) and worst-performing (right) single- and multi-echo sequences. In each panel, the top row shows TSNR for LB ON, the middle row shows LB OFF, and the bottom row shows their difference. A) Single-echo sequences. B) Multi-echo sequences. For all sequences, LB increases TSNR in the brain and reduces signal leakage outside the brain. Multi-echo sequences produce higher TSNR in ATL than single-echo sequences.
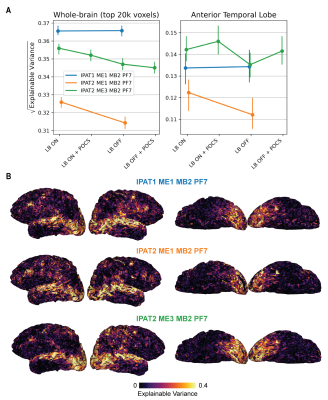
Figure 4. Sequence comparison with FSNR: explainable variance (EV). A) Median EV in a cortical (left) and ATL mask (right). The iPAT1 single-echo sequence (blue) shows higher EV in the whole brain than the other sequences, but the multi-echo sequence (green) shows higher EV in ATL. POCS reconstruction also improves EV in the ATL. B) EV on the cortical surface with LB ON. The multi-echo sequence (green) shows higher EV than the other sequences in temporal cortex.

Figure 5. Sequence comparison with FSNR: model performance (MP). A) Median model performance in a cortical (left) and ATL mask (right). The ME3 sequence (green) shows the highest model performance in the whole brain. In ATL, it performs as well as the iPAT1 single-echo sequence. POCS reconstruction with LB improves model performance in the ATL. B) Model performance on the cortical surface with LB ON. The ME3 sequence shows higher model performance than the other sequences in temporal cortex.