2680
Improved resting-state fMRI with through-plane phase precompensated spectral-spatial pulses1Radiology, Penn State College of Medicine, Hershey, PA, United States, 2Neurology, Penn State College of Medicine, Hershey, PA, United States, 3Neurosurgery, Penn State College of Medicine, Hershey, PA, United States
Synopsis
fMRI scans with long echo times are vulnerable to signal loss due to through-plane dephasing caused by susceptibility field gradients (SFG), particularly near the air-tissue interface of the sinus regions. Resting state fMRI techniques that sample networks with connectivity through these artifact-plagued regions could be compromised. Several approaches have been proposed for susceptibility compensation, with one promising approach based upon the use of phase-precompensated spectral-spatial RF pulses. These RF pulses pre-phase the magnetization to cancel out the dephasing caused by SFG’s. Here, we evaluated the ability of spectral-spatial pulses to improve detection of resting state fMRI activity.
Purpose
Resting-state fMRI (rsfMRI) samples spontaneous low-frequency fluctuations in the BOLD signal to investigate the functional topology of the brain. This technique has allowed the identification of resting state networks that demonstrate synchronized BOLD fluctuations. rsfMRI samples the whole brain, however, some structures located in the inferior part of the brain are in close proximity to air/tissue interfaces and suffer from considerable susceptibility-related MR artifacts. The through-plane signal loss due to susceptibility-induced gradients can make it difficult to detect activation in these regions. In this abstract, we investigated the use of Spectral-Spatial (SPSP) radiofrequency pulses for through-plane compensation to recover the rsfMRI signal in these regions [1,2].Methods
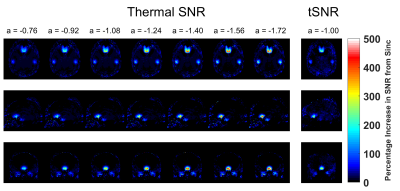
SPSP Pulse Design: The SPSP pulses were designed following the method described by Anderson et al [2]. The pulses utilized 6 frequency bands, with a frequency spacing (Δf) of 60 Hz. Six sub pulses of 2.6 ms duration were utilized, for a total pulse duration of 16 ms. The design was monopolar with flyback lobes. Fourteen SPSP pulses with α values ranging from -0.68 to -1.72 uT/m/Hz were designed through incremental shifts of the sub pulses relative to the slice select gradient center. SPSP Assessment Protocol: All SPSP pulses utilized a 70-degree flip angle. Prior to the fMRI experiment, the EPI sequence was run in a mode that swept through all 14 α values and the uncompensated sinc pulse (~40 sec acquisition time). The k-space data of this acquisition and a noise prescan were saved for further analysis. Resting-State fMRI Protocol: The rsfMRI paradigm was run twice with and without susceptibility compensation on a Siemens PrismaFit 3T with the following parameters: TR/TE = 2000 ms / 30 ms, Flip Angle = 70 deg, FOV = 220x220 mm2, matrix = 80x80, 30 axial slices, slice thickness 4 mm, parallel acceleration factor = 2, repetitions = 150. The susceptibility compensated rsfMRI scan used an α value of -1.00 uT/m/Hz for all slices, as suggested in literature [1,2] and prior work from our group [3]. Additionally, a T1w volume (1 mm3) was acquired as an anatomical scan. Data was acquired from (n =10) subjects under an IRB approved protocol. Thermal SNR & tSNR Reconstruction: The noise prescan data was used to prewhiten [4] the k-space SPSP sweep data, followed by a direct GRAPPA recon to reconstruct a SNR units map [4,5]. Thus, thermal SNR maps were available for all 14 α values and the uncompensated sinc pulse. tSNR maps were calculated from the resting-state data following detrending and realignment in SPM12. All thermal SNR & tSNR maps were coregistered to the T1w volume and then normalized to the MNI space (2 mm3 isotropic) with SPM12. Average SNR percentage improvement across all 10 subjects was calculated on a voxel-by-voxel basis from the MNI normalized data. Resting State Analysis: Amplitude of low-frequency fluctuation (ALFF) & Regional Homogeneity (ReHo) were calculated with the RESTplus tool [6]. Standard realignment, normalization, detrending were applied with the package. All data was prefiltered to 0.01 to 0.1 Hz. A paired t-test across all subjects was performed to find regions of significant difference between susceptibility compensated and uncompensated acquisitions (p < 0.05).Results and Discussion
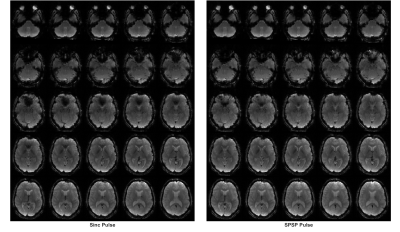
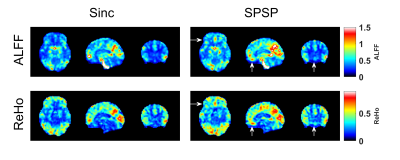
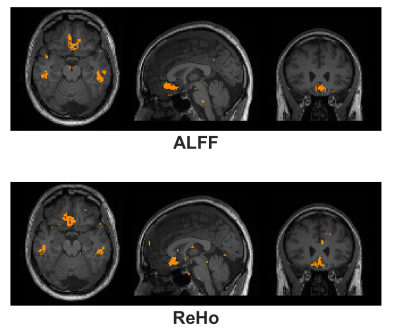
Figure 1 shows an EPI volume acquired with a standard sinc (left) and SPSP pulse (right) with α = -1.00 uT/m/Hz. Compensation is evident near the orbitofrontal cortex (OFC) region. Figure 2 displays the percentage improvement in thermal SNR & tSNR, averaged across all 10 subjects, on a voxel-by-voxel basis. For display purposes, only 7 of the 14 a values are shown. Increases of SNR range from 200 – 500% near the OFC and the sinus regions. Interestingly, the highest α value (-1.72 uT/m/Hz) achieves the greatest recovery. Figure 3 displays single-subject ALFF & ReHo maps, acquired from the same subject depicted in Fig. 1. White arrows highlight a region of strong recovery and a node that was recovered with the SPSP pulse. Figure 4 displays regions of significant difference (p < 0.05) between the compensated & uncompensated ALFF & ReHo maps, calculated across all subjects. The regions of difference correspond well with the regions of SNR increase depicted in Fig. 2. rsfMRI is a promising application for this type of SPSP RF pulse, as they are capable of restoring signal in susceptibility-plagued regions without incurring significant signal loss elsewhere in the brain. An α value of -1.00 uT/m/Hz was used in this work, but as depicted in Figure 2, a higher value could offer greater compensation.Conclusion
This work demonstrates the potential advantage of utilizing spectral-spatial pulses for susceptibility compensation when performing rsfMRI in the human brain. This work is relevant for neuroscience studies investigating rsfMRI networks with activity in susceptibility-plagued regions.Acknowledgements
No acknowledgement found.References
[1] Yip et al., “Spectral-spatial pulse design for through-plane phase precompensatory slice selection in T2*-weighted functional MRI”, MRM 2009
[2] Anderson et al., “Simultaneous Multislice Spectral-Spatial Excitations for Reduced Signal Loss Susceptibility Artifact in BOLD Functional MRI”, MRM 2014
[3] Sica et al., “Improved detection of olfactory fMRI BOLD signal with through-plane phase precompensated spectral-spatial pulses” ISMRM 2015 p.3928
[4] Kellman et al, “Image reconstruction in SNR units: a general method for SNR measurement”, MRM 2005
[5] Breuer et al. “General formulation for quantitative G‐factor calculation in GRAPPA reconstructions” MRM 2009
[6] Jia et al, “RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing”, Science Bulletin 2019
Figures