2678
Simultaneous Multi-Segment (SMSeg) EPI for Functional MRI1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Reduced field-of-view (rFOV) imaging offers several advantages, including decreased image distortion and high spatial resolution. We describe a simultaneous multi-segment (SMSeg) imaging method to extend the benefits of rFOV to multi-segment imaging in multiple focal regions. SMSeg was implemented using a 2D RF pulse whose multiple spatial response replicates excited different focal regions of interest, followed by GRAPPA reconstruction utilizing the coil spatial sensitivity variations in two orthogonal spatial directions. We have applied the SMSeg imaging technique to brain fMRI to visualize activations in multiple areas with minimal image distortion.
Introduction:
fMRI depends heavily on single-shot echo-planar imaging (ssEPI) which offers a high image acquisition speed1–3. However, the narrow sampling bandwidth along the phase-encoding direction makes ssEPI sensitive to in-plane geometric distortion. In addition, ssEPI typically has low spatial resolution. Several techniques have been proposed to address these issues. A common approach is to perform parallel imaging, which allows data under-sampling along the phase-encoding direction. The under-sampling or acceleration factor, however, is rather limited due to noise amplification and residual aliasing artifacts4. More recently, acquisition techniques based on reduced FOV (rFOV) were used in fMRI, which also reduces k-space sampling5,6. rFOV excites only a specific region of interest within a set of slices. The regions of interest (e.g., visual and motor cortices), however, are not necessarily confined to these slices. Herein, we report a novel approach to overcome this limitation in rFOV fMRI by using Simultaneous Multi-Segment (SMSeg) acquisitions with a 2D RF excitation pulse.Methods:
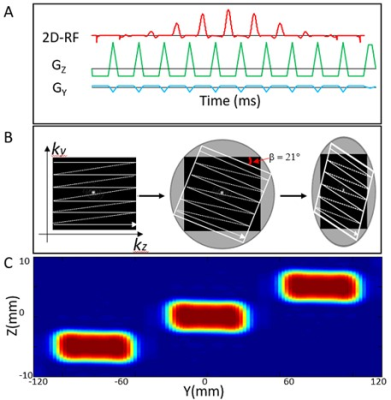
SMSeg pulse sequence design: It is well known that the spatial response of a 2D RF pulse contains a central excitation area within the plane defined by the slice-selection and phase-encoding axes and multiple replicates that can be positioned at different slice and/or phase-encoding locations5,7,8.Typically these replicates are problematic and must be avoided in sequence design. In SMSeg, however, we take advantage of these replicates to freely target different functional activation areas by using a tilted excitation k-space design irrespective of the locations of these areas of interest. A 2D RF pulse (Figure 1A) was designed by employing a fly-back EPI-like excitation k-space trajectory9,10. Eleven sub-pulses were nested under an envelope pulse with a pulse width of 21.0-28.7 ms. The replicates of the spatial response of the 2D RF pulse were freely positioned by (a) controlling the gaps between the replicates and (b) tilting the replicates (Figures 1B and 1C)9. The repositioned replicates corresponded to the segments in SMSeg imaging. For display, the non-overlapping segments (e.g., Nsegments=3) were projected onto the phase-encoding axis (Figure 2A) and combined into a single composite “slice”. The 2D RF pulse in Figure 1 was incorporated into a GRE-EPI pulse sequence for fMRI studies.Image reconstruction: Both conventional and GRAPPA11 reconstructions were applied to the simultaneously excited multiple segments, depending on whether parallel imaging was employed. Unlike conventional in-plane parallel image reconstruction, under-sampled SMSeg image reconstruction incorporated coil spatial sensitivity variations in both phase-encoding and slice-selection directions.
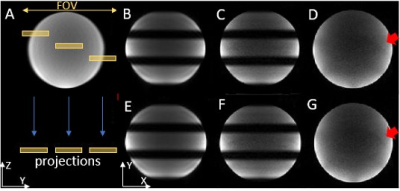
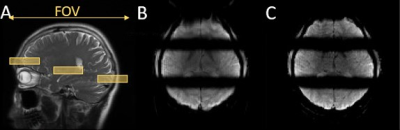
Experiments: Three experiments were conducted on a GE MR750 3T scanner using a 32-channel brain coil. In the first experiment, a water phantom was used to validate the SMSeg technique with the following protocol: excitation k-space tilt angle $$$\beta$$$ = 21° (Figure 1B), Nsegments = 3, FOV = 200x200 mm2, in-plane spatial resolution = 1.67x1.67 mm2, slice thickness/spacing = 4/1 mm, number of slices = 10, TR/TE = 2000/30 ms, and scan time = 2 s. Further, the feasibility of combining SMSeg with parallel imaging was evaluated using an acceleration factor of 4. For comparison, a one-segment conventional EPI acquisition was performed at the central segment location with the same parameters as in the accelerated SMSeg acquisition. In the second experiment, the same imaging protocol for the phantom was applied to brain imaging of a healthy human subject except for FOV = 240x240 mm2. The third experiment was to demonstrate the feasibility of using SMSeg EPI for fMRI in multiple focal areas including both visual and motor cortices. Since these areas spanned a limited FOV along the phase-encoding direction, a smaller FOV of 240x120 mm2 was used to illustrate the flexibility of SMSeg in FOV selection (Figure 4A). The imaging parameters were: TR/TE = 80/32 ms, flip angle = 30°, acceleration factor = 2, matrix size = 120x30, in-plane resolution = 2x2 mm2, slice thickness = 4 mm, and tilt angle $$$\beta$$$ = 70°. A block-design visuomotor simulation task was used. All data were analyzed using SPM8 on MATLAB 2012b.
Results:
The right panel of images in Figure 2 shows the phantom results in which the targeted three segments were successfully obtained, as displayed in two representative composite slices from the non-accelerated acquisition (B and E). With a 4-fold GRAPPA acceleration, the SMSeg image quality (C and F) outperformed that of the conventional GRAPPA images (D and G) where residual aliasing artifacts and noise amplification were more pronounced. Human brain imaging results in Figure 3 illustrated compatibility of SMSeg with GRAPPA with a relatively high acceleration factor over a reduced FOV. Figure 4 displays the fMRI experimental results in which activations in different areas (i.e., visual cortex, primary motor cortex, and supplementary motor area) were visible in two segments in a single composite slice.Discussion and conclusion:
We have demonstrated a novel SMSeg technique that simultaneously excites multiple segments in different slices and/or phase-encoding locations to target specific functional regions in the brain. The segments can benefit from reduced FOV imaging, such as decreased image distortion and improved spatial resolution. The SMSeg approach is also compatible with parallel imaging and provides additional robustness against residual aliasing artifacts arising from parallel image reconstruction.Acknowledgements
This work was supported in part by the National Institutes of Health (5R01EB026716-01 and 1S10RR028898-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992.
2. Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992.
3. Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med. 1992.
4. De Zwart JA, van Gelderen P, Golay X, et al. Accelerated parallel imaging for functional imaging of the human brain. NMR Biomed. 2006.
5. Finsterbusch J. Functional neuroimaging of inner fields-of-view with 2D-selective RF excitations. Magn Reson Imaging. 2013.
6. Islam H, Glover GH. Reduced field of view imaging using a static second-order gradient for functional MRI applications. Magn Reson Med. 2016.
7. Saritas EU, Cunningham CH, Lee JH, et al. Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008.
8. Alley MT, Pauly JM, Sommer FG, et al. Angiographic imaging with 2D RF pulses. Magn Reson Med. 1997.
9. Finsterbusch J. Fast-spin-echo imaging of inner fields-of-view with 2D-selective RF excitations. J Magn Reson Imaging. 2010.
10. Zhong Z, Merkitch D, Karaman MM, et al. High-spatial-resolution diffusion MRI in Parkinson disease: Lateral asymmetry of the substantia nigra. Radiology. 2019.
11. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn Reson Med. 2002.
Figures