2676
The vessel size specificity and sensitivity of rapid CPMG sequences in functional BOLD imaging
Klaus Scheffler1,2, Joern Engelmann1, and Rahel Heule1,2
1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2University of Tuebingen, Tuebingen, Germany
1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2University of Tuebingen, Tuebingen, Germany
Synopsis
For reduced refocusing flip angles, the peak of the vessel size sensitivity curve is shifting towards larger radii with increasing echo time. The BOLD sensitivity is largely independent of the refocusing flip angle down to about 40°. CPMG or GRASE can be used with low refocusing flip angles without significant loss of sensitivity to BOLD and without the need for centric reordering. Signals acquired before or after the spin echo time point show contributions from larger vessels similar to gradient echo sequences. This effect is reduced for longer echo times.
Introduction
While the vessel size specificity of gradient and spin echoes has been extensively characterized in simulations and measurements, other sequence types that recently gain increasing attention in ultra-high field and high-resolution BOLD fMRI such as CPMG and GRASE are yet not fully characterized (1-2). CPMG or its variant GRASE that samples a gradient echo train within consecutive (gradient and rf) refocusing pulses offers a simple possibility for inner volume acquisition by using perpendicular excitation and refocusing slice orientations which is beneficial for confined high-resolution BOLD measurements (3-6). Furthermore, CPMG or GRASE offers several sequence parameters such as variable refocusing flip angles, echo spacing and k-space reordering schemes along the gradient and spin echo train that might influence its sensitivity to certain vessel radii. In this contribution, we present an analysis of the vessel size specificity of CPMG (and GRASE) sequences across field strength, for different echo spacings, reduced and varying refocusing flip angles, and for acquisition time points before and after the spin echo to assess gradient echo related contributions. Results are based on Monte Carlo simulations of extravascular signals disturbed by randomly oriented cylinders with different radius as well as with measurements on microspheres.Methods
The calculation of the extravascular signal was done similarly as described in previous papers, except that a linear evolution of the spin phase was added during periods of free precession and random walks to represent higher order configurations that appear if refocusing pulses with reduced flip angle are applied. Magnetization was represented by 512 independent magnetization vectors that are in-phase after the initial 90° excitation pulse. During free precession until the first refocusing pulse, this set of magnetization vectors see a linear phase evolution up to ±p resulting in a net transverse magnetization (sum over this set of spins) of zero. During the following consecutive refocusing pulses a linear dephasing of ±2p is applied leading to a spin echo between the first and second refocusing pulse, and a combination of higher echoes after the second refocusing pulse. For the experimental validation of the Monte Carlo simulation results, MR measurements on precision polybead polystyrene microspheres (Polysciences Europe) of varying diameters and Dc = 0.1 relative to water were performed at 3T (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany), similar as described in Bieri and Scheffler (7), using the 64‐channel receive head array coil of the manufacturer.Results
Fig. 1a shows an example of the vessel size specificity simulated for 3T with an echo spacing of 10 ms and echo time of 100 ms corresponding to the 10th echo along the CPMG train for refocusing flip angles from 20° to 180°. A reduction of the refocusing flip angle causes a shift of the plots towards larger vessel radii. These findings are corroborated experimentally by the results obtained from CPMG acquisitions with different refocusing flip angles at 3T in test tubes containing microspheres with different diameters (Fig. 1b). The simulations in Fig. 2 top row show vessel radius with strongest signal change DMmax for different refocusing flip angles along the CPMG echo train for an echo spacing of 15 ms and for different field strength. For low refocusing flip angles the vessel radius at DMmax increases along the echo train as these echoes are composed of higher-order echoes with increasingly longer spin history. Fig. 2 bottom row depicts the mean signal change (DMmean is the mean signal change along vessel size radius from 0.5 to 300 mm indicating the overall BOLD response) along the echo train for different constant refocusing flip angles. In addition, variable refocusing flip angle trains with target echo amplitudes of 0.1, 0.2 and 0.3 M0 are shown as dashed black lines. The vessel size specificity of asymmetric echoes is a mixture of the spin echo and gradient echo profiles, as demonstrated in Fig. 3a. Just before application of the first refocusing pulse, the vessel size specificity is identical to a gradient echo that shows an unspecific and constant signal change for all vessels larger than about 3-5 mm. A pure spin echo response is visible at the center between refocusing pulses with an initially increasing signal change for early echoes that peaks around TE=60ms and decays for later echoes. Finally, mean signal changes along longer GRASE echo trains and echo spacings as used in the papers of Kemper et. al. (6, 8) with variable, and Beckett et. al. (9) with constant refocusing flip angles are shown in Fig. 3b.Acknowledgements
This work was funded in part by DFG, a Reinhard Koselleck Project, DFG SCHE 658/12, and by the Max Planck Society.References
1. Constable RT, Kennan RP, Puce A, McCarthy G, Gore JC. Functional NMR imaging using fast spin echo at 1.5 T. Magn Reson Med 1994;31:686–690. 2. Poser BA, Norris DG. Fast spin echo sequences for bold functional MRI. Magma 2007; 20 (1), 11–17. 3. Feinberg DA, Harel N, Ramanna S, Ugurbil K, Yacoub E. Sub-millimeter single-shot 3D GRASE with inner volume selection for T2 weighted fMRI applications at 7 Tesla. Proceedings 16th Annual Meeting International Society for Magnetic Resonance in Medicine, 2008:p. 2373. 4. Zimmermann J, Goebel R, De Martino F, van de Moortele PF, Feinberg D, Adriany G, et al. (2011). Mapping the organization of axis of motion selective features in human area MT using high-field fMRI. PLoS ONE 6:e28716. doi: 10.1371/journal.pone.0028716 5. De Martino F, Zimmermann J, Muckli L, Ugurbil K, Yacoub E, Goebel R. (2013). Cortical depth dependent functional responses in humans at 7T: improved specificity with 3D GRASE. PLoS ONE 8:e60514. doi: 10.1371/journal.pone.0060514 6. Kemper VG, De Martino F, Vu AT, Poser BA, Feinberg DA, Goebel R, Yacoub E. Sub-millimeter T2 weighted fMRI at 7 T: comparison of 3D-GRASE and 2D SE-EPI. Front. Neurosci. 9:163.doi: 10.3389/fnins.2015.00163 7. Bieri, O., Scheffler, K. Effect of diffusion in inhomogeneous magnetic fields on balanced steady-state free precession. NMR Biomed. 2007. 20, 1–10. 8. Kemper VG, De Martino F, Yacoub E, Goebel R. Variable flip angle 3D-GRASE for high resolution fMRI at 7 T. Magn Reson Med. 2016 September; 76(3): 897–904. doi:10.1002/mrm.25979. 9. Beckett AJS, Dadakova T, Townsend J, Huber L, Park S, Feinberg DA. Comparison of BOLD and CBV using 3D EPI and 3D GRASE for cortical layer functional MRI at 7 T. Magn Reson Med. 2020 Jun 18. doi: 10.1002/mrm.28347. Online ahead of print. PMID: 32557752. 10. Busse RF,Hariharan H,Vu A,Brittain JH. Fast Spin Echo Sequences With Very Long Echo Trains: Design of Variable Refocusing Flip Angle Schedules and Generation of Clinical T2 Contrast. Magnetic Resonance in Medicine 55:1030–1037 (2006).Figures
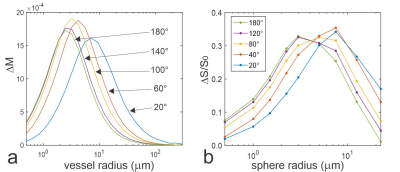
Fig. 1. a: Vessel size dependent signal change
simulated at 3T for a multi echo CPMG sequence for constant refocusing flip
angles from 20 to 180 degrees at echo time TE = 100 ms and echo spacing of 10
ms (i.e. signal of 10th echo). b: CPMG experiments at 3T with 64
echoes separated by an echo spacing of ΔTE = 6 ms on microspheres of diameters ranging
from 1 to 45 µm.
Relative signal intensities ΔS/S0
(S0: reference test tube without microspheres) are plotted versus
sphere radius at TEmax = 384 ms for constant refocusing flip angles
of 20°, 40°, 80°, 120°, and
180°.
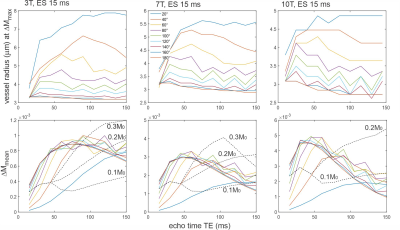
Fig. 2. Top row: Size of vessel radius with maximal
signal change DM0 as a function of echo time and field strength
and refocusing flip angles. For small refocusing pulses the vessel radius with
maximal signal change increases with echo number. Bottom row: mean signal
change (averaged across vessel radius) as a function of constant refocusing
flip angles from 20° to 180°, and for varying refocusing flip angles with
target echo amplitudes of 0.1, 0.2 and 0.3 M0. These flip angles were
calculated for field strength dependent relaxation times according to the
algorithm given by Busse (10).
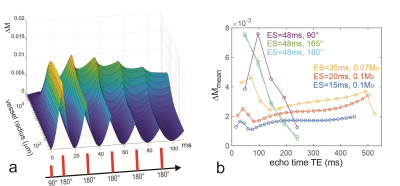
Fig 3. a: Signal change as a function of vessel size
calculated in time steps of 0.4 ms along a multi-echo spin echo train with
perfect 180° refocusing pulse at 7T with echo spacings of 20 ms. The (gradient
echo-like) profiles immediately before and after later refocusing show
increasingly less sensitivity to larger vessels. b: Mean signal changes along
GRASE echo trains at 7T using echo spacings of 15, 20, 35 with variable
refocusing flip angles that target an echo amplitude of 0.1 and 0.07 M0
(6, 8) and 48 ms echo spacing with constant refocusing flip angles of 165° (9)
as well as 90° and 180°.