2675
Recommendations of choice of head coil and prescan normalize filter depend on region of interest and task1Neuroimaging Unit, School of Medicine and Health Sciences, Carl-von-Ossietzky University of Oldenburg, Oldenburg, Germany, 2Neuroimaging Unit, Carl-von-Ossietzky University of Oldenburg, School of Medicine and Health Sciences, Oldenburg, Germany, 3Applied Neurocognitive Psychology, Department of Psychology, School of Medicine and Health Sciences, Carl-von-Ossietzky University of Oldenburg, Oldenburg, Germany, 4Cluster of Excellence “Hearing4all”, Carl-von-Ossietzky University of Oldenburg, Oldenburg, Germany
Synopsis
The performance of the 20- and the 64-channel head coil and the influence of the prescan normalize filter was evaluated in a standard fMRI setting using a 3T Siemens Prisma MRI. Larger beta estimates and tSNR occurred in auditory cortex and thalamus with prescan normalize, in contrast to the visual and motor cortex with larger beta estimates and tSNR without prescan normalize. In line with the results of the MRIQC tool, the 20-channel head coil with prescan normalize is better suitable for functional measurements, especially in deeper brain areas. The 64-channel head coil is the best choice for anatomical scans.
INTRODUCTION
For (f)MRI a variety of head coils are available with various geometries and different number of channels. In the current study, the differences in performance between the 20-channel and 64-channel head coil provided by Siemens for 3T MRI machines together with the influence of the prescan normalize filter was investigated using a standard fMRI setting (echo-planar imaging) with three different tasks (auditory, visual and motor) known to activate different areas in the brain with various distances from the head coil surfaces. MR coil signals can be compared in various aspects, such as the (time-course) signal-to-noise-ratio (tSNR) which is assumed to increase with increasing number of channels, but also decreases with increasing distance to the coil surface [1–6] or the blood oxygenation level dependent (BOLD) signal [5, 4, 7, 8]. Additionally, the signal quality related to head coils and the influence of the prescan normalize was investigated with a structural T1 MPRAGE sequence. The performance of the different head coils and the influence of the prescan normalize filter is systematically analyzed with the MRIQC tool [9] in functional a well as structural sequences.METHODS
Written informed consent was obtained from 26 right-handed healthy volunteers (12 males; age range 19-31 years). Three participants were removed due to head movements or non-compliance with the task instruction. Three different tasks were used. A motor, an auditory, and a visual task, each consisting of a 20 second stimulus block was followed by a 20 second rest block in which only a fixation cross was presented, both repeated eight times. Each task was repeated twice, with and without prescan normalize, followed by a structural T1-weighted MPRAGE image. After changing of the head coil the same tasks and the T1-weighted image were repeated. The MRIQC software [9] was used to evaluate image quality metrics (IQMs) for the functional and structural data. The IQMs were compared with ANOVAs. After calculation of the GLM for each participant, the contrast beta estimates for the highest activated voxel of each ROI (auditory cortex, visual cortex, motor cortex and thalamus) were calculated. To evaluate the time-course SNR (tSNR) the mean tSNR was extracted in each voxel in the given ROI. Both, the results of the beta estimates and tSNR were analyzed in ANOVAs.RESULTS
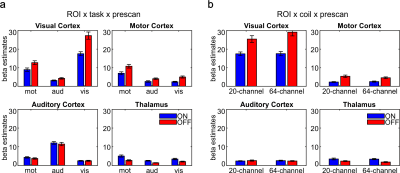
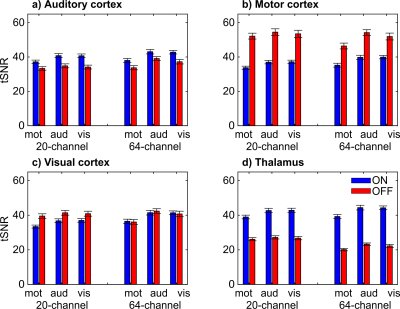
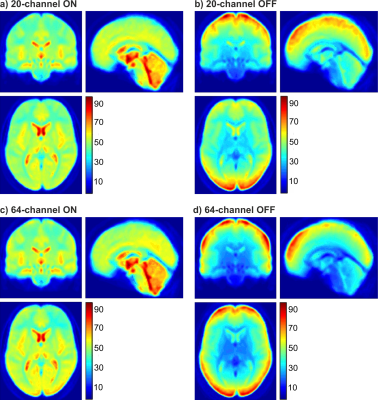
The IQMs indicated better results with prescan normalize for functional data with the 20-channel and for structural data with the 64-channel head coil. The ANOVA for the beta estimates revealed no difference in head coils but an influence of prescan normalize, F(1,22) = 78.64, p < .001, with larger beta estimates without prescan normalize. The performance was influenced by the choice of task and ROI as shown in the interaction ROI x task x prescan normalize, F(6,132) = 22.82, p < .001 (Figure 1A) and ROI x head coil x prescan normalize, F(3,66) = 4.93, p = .02 (Figure 1B). TSNR showed a significant overall interaction, F(6,132) = 5.65, p = .004. Separate ANOVAs for each ROI (Figure 2) indicated in the auditory ROI, larger tSNR in the 64-channel head coil with prescan normalize. In the motor ROI, there was no difference between head coils, but larger tSNR without prescan normalize. In the visual ROI, there were larger tSNR in the 64-channel head coil without prescan normalize. In the thalamus, however, tSNR was stronger in the 20-channel head coil with prescan normalize. The normalized distribution of the tSNR is shown in Figure 3.DISCUSSION
For standard fMRI analyses, a recommendation of head coil and prescan normalize strongly depends on the task and the ROI. Studies [7] and [8] showed higher activation in those head coils with more channels, mainly in the superficial but not in deeper regions, which could be confirmed by the current study as well, mainly for the 64-channel head coil in the visual cortex. Study [7] reported that the prescan normalize filter affected beta estimates in the 32-channel head coil mainly in deeper regions but not in the 12-channel coil. In contrast, we found no difference between the 20- and the 64-channel coils and a more distinct pattern with enhanced activation in the deeper regions and auditory areas with prescan normalize on, replicating [7], but enhanced activation in the visual and motor cortex without prescan normalize. tSNR differs between head coils and prescan normalize depending on task and ROI. The results are mostly in line with the results of beta estimates. The visualization of the overall pattern of the tSNR (Figure 3) support the ROI based findings that prescan normalize enhances the tSNR in the deep brain compartments. For cortical regions we could not see a clear improvement in tSNR, except for the 64-channel head coil in the occipital lobe.CONCLUSION
For studies investigating subcortical brain areas and the auditory cortex, we would recommend to use the prescan normalize filter, whereas it seems not necessary in the visual and motor cortex. Compared to the prescan normalize filter the coils have only little effect.Acknowledgements
This work was supported by the Neuroimaging Unit of the Carl-von-Ossietzky Universität Oldenburg funded by grants from the German Research Foundation (3T MRI INST 184/152-1FUGG).References
1. Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla. Design optimization and evaluation. Magnetic Resonance in Medicine 2009:62:754–762.
2. Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magnetic Resonance in Medicine 2006:56:216–223.
3. Zwart JA de, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magnetic Resonance in Medicine 2004:51:22–26.
4. Albrecht J, Burke M, Haegler K, et al. Potential impact of a 32-channel receiving head coil technology on the results of a functional MRI Paradigm. Clin Neuroradiol 2010:20:223–229.
5. Keil B, Blau JN, Biber S, et al. A 64-channel 3T array coil for accelerated brain MRI. Magnetic Resonance in Medicine 2013:70:248–258.
6. Reiss-Zimmermann M, Gutberlet M, Köstler H, Fritzsch D, Hoffmann K-T. Improvement of SNR and acquisition acceleration using a 32-channel head coil compared to a 12-channel head coil at 3T. Acta Radiol 2013:54:702–708.
7. Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil. Are there relevant improvements for functional imaging? Journal of Magnetic Resonance Imaging : JMRI 2011:34:173–183.
8. Kahn I, Wiggins CJ, Wiggins G, et al. High-resolution human functional MRI. Feasibility and specificity at high (3T) and ultrahigh (7T) fields. In: Proceedings of the Joint Annual Meeting ISMRM-ESMRMB, Berlin; 2007.
9. Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ, Bernhardt BC. MRIQC. Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS ONE 2017:12:e0184661.
Figures