2668
Evaluation of a Deep Learning reconstruction framework for three-dimensional cardiac imaging
Gaspar Delso1, Marc Lebel2, Suryanarayanan Kaushik2, Graeme McKinnon2, Paz Garre3, Pere Pujol3, Daniel Lorenzatti3, José T Ortiz3, Susanna Prat3, Adelina Doltra3, Rosario J Perea3, Teresa M Caralt3, Lluis Mont3, and Marta Sitges3
1GE Healthcare, Barcelona, Spain, 2GE Healthcare, Waukesha, WI, United States, 3Hospital Clínic de Barcelona, Barcelona, Spain
1GE Healthcare, Barcelona, Spain, 2GE Healthcare, Waukesha, WI, United States, 3Hospital Clínic de Barcelona, Barcelona, Spain
Synopsis
In this study we evaluate a Deep Learning reconstruction framework with adjustable noise reduction on a database of clinical cases, including Delayed Myocardial Enhancement (MDE), Phase-Sensitive MDE (PSIR) and 3D Heart datasets.
Introduction
Three-dimensional imaging sequences facilitate the understanding of complex pathological patterns in cardiac imaging, both of function and morphology. They enable full heart coverage with more isotropic resolutions than their two-dimensional counterparts, albeit at the price of increased acquisition time, especially for patients with irregular cardiorespiratory patterns.Deep Learning (DL) methods that learn how to reconstruct the image from training on previous data have shown great potential for medical imaging. A reconstruction method that makes more efficient use of the acquired data to produce sharp, high signal-to-noise (SNR) images would substantially benefit cardiac 3D acquisitions, adding more flexibility in protocol adjustments and enabling better leveraging of available acceleration techniques such as parallel and partial Fourier imaging.
In this study, we evaluate a novel DL reconstruction framework, designed to provide user-tuneable noise reduction, improve edge sharpness and reduce truncation artifacts, on a database of cardiac MRI clinical cases, including Delayed Myocardial Enhancement1 (MDE), Phase Sensitive MDE (PSIR) and 3D angiography (MRA) datasets.
Methods
A set of 37 cases (29 male, 8 female, age 51±18 years, weight 82±14 kg) were selected for this study. All subjects had been referred to Hospital Clínic of Barcelona for a contrast-enhanced cardiac examination on a GE Architect 3T MRI.In each case, the protocol included one or more 3D series. The typical Delayed Myocardial Enhancement acquisition settings were: SPGR readout with IR preparation, fat suppression, spatial saturation, respiratory navigator, FOV 40x40cm2, 320x320x100, ST 2.4mm, FA 20deg, BW 62.5 kHz, TE min full, 2x ARC acceleration. The inversion time was selected using CINE IR scout series, to achieve either myocardium or blood nulling. The MR Angiography acquisitions used the same underlying sequence with slightly different settings: FOV 38x38cm2, 288x288x56, ST 3.0mm, FA 15deg, BW 125 kHz, TE min full, 2x ASSET acceleration.
The raw data of all acquisitions was exported for offline processing. A reference reconstruction was obtained, using 3D Cartesian pipeline equivalent to the one in the scanner. A second reconstruction was obtained using a deep convolutional network that operates on complex-valued data to produce sharpened and de-noised output. The architecture is intensity invariant, compatible with all relevant image sizes and suitable for blind denoising of arbitrary amplitude, including spatially variant noise2. The algorithm was implemented using Python, Google’s TensorFlow library and GE’s Orchestra library. Two regularization settings were tested in each case (0.75 and 1.00).
The reconstructions were reviewed by a board-certified cardiologist for discrepancies of diagnostic relevance. The structural similarity index (SSIM) and voxel-wise relative standard deviation (RSD) were used to quantify noise reduction and structure preservation. Delayed enhancement datasets were processed with ADAS arrhythmogenic tissue analysis software (ADAS3D Medical S.L.)
Results
The Deep Learning framework successfully reconstructed all the collected datasets. Illustrative examples of the results can be found for 3D MDE series (figure 1), phase sensitive MDE series (figure 2) and 3D angiography series (figure 3). Reconstruction times were under 2 minutes on an Intel Xeon Silver 4116 CPU using a NVIDIA Tesla P40 GPU.As can be appreciated in the joint RSD histograms of figure 4, the DL method consistently improved the SNR of the reconstructed images. This was particularly evident in coronal and sagittal planes. Profile analysis showed no evidence of edge degradation in the regularized images, with a marginal reduction of truncation artifacts.
The clinical evaluation of the results didn’t reveal any cases where the DL reconstruction led to a loss of structures of interest, or to an alteration of contrast uptake pattern that would alter the diagnostic outcome. The available data were insufficient to determine whether DL images improved lesion detectability.
DL reconstruction didn’t affect the segmentation or quantitative metrics yielded by ADAS. Areas identified as pathological overlapped on both reconstructions, as illustrated on Figure 5.
Discussion
The Deep Learning framework invariably provided improved image quality compared to the Cartesian reconstruction currently used in clinical practice. This was achieved with reconstruction times smaller than the corresponding acquisition times, suggesting that routine clinical use is possible without saturating the reconstruction queue.No new artifacts were found to be caused by the DL reconstructions, but artifacts typically seen in the Cartesian images (e.g. cardiac and respiratory motion) were not eliminated either. This behaviour seems to extend to image feature preservation, with all morphological and functional structures of diagnostic relevance being preserved by the algorithm.
The appreciation of the benefits of DL reconstruction is limited by the retrospective nature of this study, with acquisitions optimized for Cartesian reconstruction. As DL reconstruction can remove the noise generated by acceleration methods (e.g. parallel imaging and partial Fourier), it enables the optimization of protocols for reduced scan time without loss of image quality. A prospective study should show whether this could lead to a reduction of artifacts related to arrhythmic cardiorespiratory motion.
Conclusion
The results suggest that the Deep Learning framework can be readily applied for the reconstruction of cardiac 3D sequences. It provides consistent image quality enhancement, with clinically acceptable reconstruction times and no evidence of artifacts or loss of diagnostically relevant structures.Future work will focus on extending the test database with prospective acquisitions, exploring the potential of DL reconstruction for protocol optimization and scan time reduction.
Acknowledgements
No acknowledgement found.References
1. Dulce, M. C. et al. MR imaging of the myocardium using nonionic contrast medium: signal-intensity changes in patients with subacute myocardial infarction. AJR Am J Roentgenol 160, 963–970 (1993).
2. Lebel, R. M. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv:2008.06559 [cs, eess] (2020).
Figures
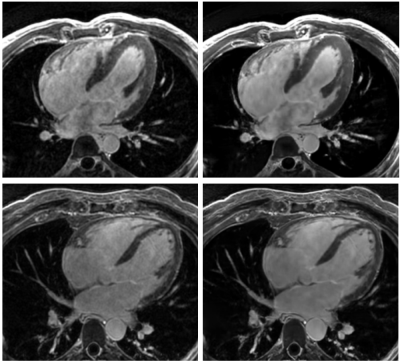
Figure
1.- Long axis views of 3D MDE series, reconstructed
with a standard 3D Cartesian method (left) and the proposed Deep Learning
framework (right).
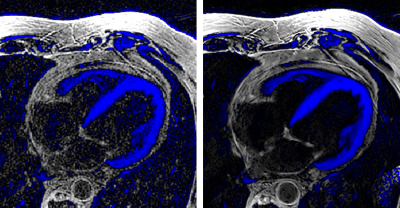
Figure
2.- Axial views of phase sensitive, black blood 3D
MDE series, reconstructed with a standard 3D Cartesian method (left) and the
proposed Deep Learning framework (right). Blue areas indicate negative
intensity values.
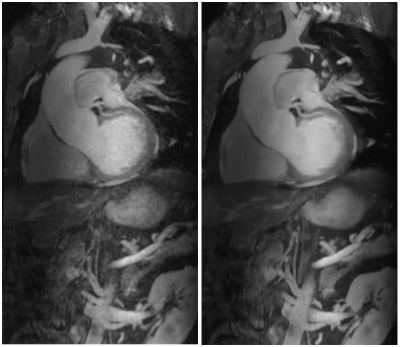
Figure
3.- Coronal maximum intensity projections of a 3D
MRA series, reconstructed with a standard 3D Cartesian method (left) and the
proposed Deep Learning framework (right).
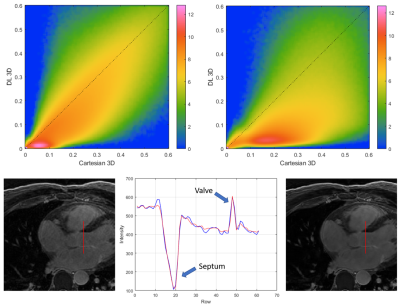
Figure
4.- Top: Logarithmic joint histograms of the
voxel-wise relative standard deviation, in the reference Cartesian and DL
reconstructions shown in figure 1. Notice how most voxels are located below the
identity line, indicating SNR improvement. Bottom: Line profile illustrating
the preservation of structure edges with the regularized reconstruction.
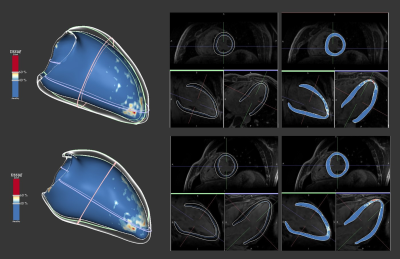
Figure
5.- Three-plane view and ADAS 3D analysis of an MDE
dataset, reconstructed with 3D Cartesian (top) and the new DL framework
(bottom).