2666
AI-based Computer-Aided System for Cardiovascular Disease Evaluation (AI-CASCADE) for carotid tissue quantification1Bioengineering, University of Washington, Seattle, WA, United States, 2Electrical Engineering, University of Washington, Seattle, WA, United States, 3Radiology, University of Washington, Seattle, WA, United States, 4Biomedical Engineering, Tsinghua University, Beijing, China, 5Surgery, University of Washington, Seattle, WA, United States
Synopsis
The tissue composition of carotid atherosclerotic plaques is crucial for cardiovascular risk assessment and can be quantified with high-resolution multi-contrast MRI by expert reviewers. The purpose of this work is to develop AI-CASCADE, a fully automated solution for quantitative analysis of carotid MRI, including artery localization, vessel wall segmentation, artery registration and plaque component segmentation. Results in our preliminary study show that AI-CASCADE achieves good agreements with manual results and has great potential as an efficient and reliable clinical tool.
Introduction
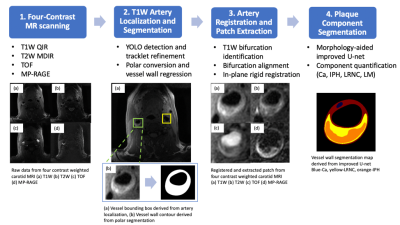
Atherosclerotic plaque rupture of the carotid artery is a major cause of ischemic stroke [1]. In recent years, high-resolution multi-contrast magnetic resonance imaging (MRI) has emerged as a promising tool for visualization of carotid atherosclerotic plaques, and has been proved to be reproducible for tissue composition quantification [2-3]. In a previous work [4], an automatic CNN-based algorithm was introduced to segment plaque tissues from localized, registered patches of T1W, T2W, TOF and MP-RAGE images. However, to generate such patches, it still requires a series of pre-processing steps by expert reviewers, which includes identifying the artery and registering images of different contrast weighting. In this work, we aimed to develop AI-CASCADE (AI-based Computer-Aided System for CArdiovascular Disease Evaluation), a fully automated workflow for quantitative carotid plaque analysis, including artery localization, vessel wall segmentation, artery registration and plaque component segmentation. The detailed workflow of AI-CASDCADE is shown in Fig 1.Methods
Data: We used carotid images of 1098 subjects from the Chinese Atherosclerosis Risk Evaluation study (CARE II) [5]. Subjects with recent stroke and/or transient ischemic attack and evidence of carotid atherosclerosis were recruited for the study. All MR imaging was performed on 3T MR scanners with an 8-channel phase array coil, using identical imaging protocol. Detailed scanning parameters are summarized in [5]. Manual analysis using CASCADE [6] was performed by experienced reviewers at University of Washington Vascular Imaging Lab (VIL), which included selecting regions of interest in the bilateral carotid arteries, aligning images manually, and outlining and labeling tissue components. 20% of the subjects were randomly selected as a holdout test set in this study.Our new algorithm includes the following steps:
1. Artery localization and segmentation with T1W images [7]: A vessel detector followed by tracklet refinement was developed to first identify the region of interest centered on lumen along T1W image slices. Then, the vessel wall contours (lumen and outer wall boundaries) were automatically traced using a polar conversion segmentation algorithm.
2. Artery registration and patch extraction: The location of carotid bifurcation was defined as the slice with the least circularity in lumen contours. The multi-contrast registration was first performed with a z-direction bifurcation alignment. Circular registration vessel masks [8], of which center and radius determined by the lumen center and outer wall boundary respectively, were generated at five consecutive slices around the bifurcation slices. X,y-plane rigid registrations followed. Mutual information was used as the matching criteria.
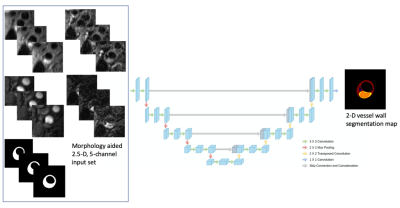
3. Plaque component segmentation: The input of the improved U-net is a morphology-aided 2.5-D, 5-channel extracted image patches, as shown in Fig 2, where the center slice of three is the target to be segmented, and vessel wall contours provide additional morphological information, as the 5th channel besides the four contrast weightings. The output is a 2-D map with regions of calcification (Ca), intraplaque hemorrhage (IPH), lipid-rich necrotic core (LRNC) and loose matrix (LM). Network structure, loss design and training paradigms are detailed in [4].
Results
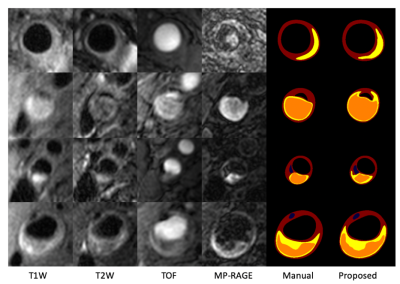
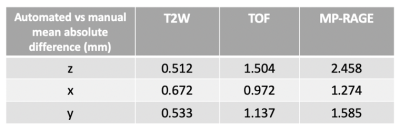
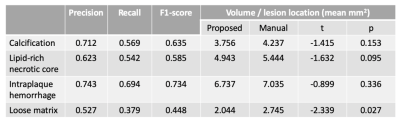
The performance of each procedure in the workflow were evaluated. (1) The accuracy of bifurcation slice identification is 88.7%. (2) Using manual registration as ground truth, the proposed automated registration shows good consistency with expert reviewers, with less than 2.5mm of mean absolute difference in any contrasts from any of the axes (Table 1). The discrepancy is slightly larger for MP-RAGE due to its low signal-to-noise ratio. (3) The proposed composition segmentation method demonstrated satisfactory agreement with manual results, as shown in Fig 3. Evaluation of tissue segmentation accuracy is detailed in Table 2. We have already shown the superiority over the Bayesian method MEPPS [9] in a previous study [4]. Moreover, Table 2 summarized the tissue area measurement per lesion location. Although the measured area of Ca, IPH and LRNC were smaller than the manual results, the differences were not statistically significant (p>0.05).Conclusion
AI-CASCADE, a fully automated solution for quantitative analysis of carotid MRI, achieves good agreement with manual results from expert reviewers and has great potential as an efficient and reliable clinical tool.Acknowledgements
The authors wish to acknowledge all the reviewers and CARE II investigators for their work in data collection and annotation.References
[1] Atherosclerosis (2017, Apr 30). Retrieved from http://www.heart.org/en/health-topics/cholesterol/about-cholesterol/atherosclerosis
[2] Cai, Jian-Ming, et al. "Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging." Circulation 106.11 (2002): 1368-1373.
[3] Saam, Tobias, et al. "Quantitative evaluation of carotid plaque composition by in vivo MRI." Arteriosclerosis, thrombosis, and vascular biology 25.1 (2005): 234-239.
[4] Guo, Yin, et al. "Carotid plaque composition segmentation in multi-contrast MRI with U-net." Proceedings of ISMRM 2019.
[5] Zhao, Xihai, et al. "Chinese Atherosclerosis Risk Evaluation (CARE II) study: a novel cross-sectional, multicentre study of the prevalence of high-risk atherosclerotic carotid plaque in Chinese patients with ischaemic cerebrovascular events—design and rationale." Stroke and Vascular Neurology 2.1 (2017): 15-20.
[6] Kerwin, William, et al. "Magnetic resonance imaging of carotid atherosclerosis: plaque analysis." Topics in Magnetic Resonance Imaging 18.5 (2007): 371-378.
[7] Chen, Li, et al. "Automated Artery Localization and Vessel Wall Segmentation using Tracklet Refinement and Polar Conversion." IEEE Access (2020).
[8] van ˈt Klooster, R., et al. "Automated registration of multispectral MR vessel wall images of the carotid artery." Medical physics 40.12 (2013): 121904.
[9] Liu, Fei, et al. "Automated in vivo segmentation of carotid plaque MRI with morphology‐enhanced probability maps." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55.3 (2006): 659-668.
Figures