2657
Fully Automated Myocardium Strain Analysis using Deep Learning1United Imaging Intelligence, Cambridge, MA, United States, 2UIH America, Inc., Houston, TX, United States, 3Cardiology, Washington University School of Medicine, St. Louis, MO, United States
Synopsis
Myocardium strain measures myocardial deformation and has been demonstrated a comprehensive, sensitive and early indicator of cardiac dysfunction. Feature tracking can assess myocardium strain from cine CMR images with no special acquisitions but requires observer intervention and expertise. We propose a deep-learning-based fully-automated myocardium strain assessment system that requires zero human intervention for accurate strain assessment.
Introduction
Myocardium strain measures myocardial deformation and has been demonstrated a comprehensive, sensitive and early indicator of cardiac dysfunction [1-3]. One of the roadblocks for a broad clinical application is the complicated data acquisition and post-analysis process in myocardium strain analysis. Recently, feature-tracking (FT) has been applied to clinical routine cine CMR images, which requires no special acquisitions like in tagging [4], DENSE [5] and SENC [6]. Considerable effort has been focused on increasing the accuracy and reproducibility of FT strain assessment but the process still requires human intervention. It has been shown that the performance and reproducibility of FT is directly related to observer's experience [14]. In this study, we propose a deep-learning-based fully-automated myocardium strain assessment system (autoFT) that can provide global and segmental strain estimates directly from cine CMR images with zero human intervention. The system was validated on patient data and compared to fast-strain-encoded (fastSENC) imaging [7].Methods
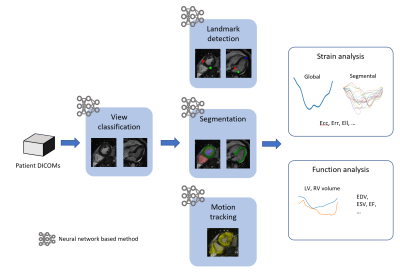
The proposed system and workflow are illustrated in Fig.1 and briefly introduced as follows: View classification: a VGG-like neural network (NN) [8] was trained to classify and group DICOM images into short-axis (SAX) stacks, 2-chamber, 3-chamber and 4-chamber long-axis (LAX) images. Landmark detection: a UNet-like NN [9] was trained to detect anatomical landmarks (RV-LV insertion points for SAX, mitral annulus and LV apical tip for LAX) on images. The landmarks were used to define the AHA 16 segments. Segmentation: a UNet-like NN was trained to segment LV myocardium on all cardiac phases and slices. Motion tracking: a motion-pyramid NN was trained to predict dense motion field between two consecutive images (not segmentation masks) [10-12]. The NN was trained in an unsupervised manner with anatomy awareness and self-adapting capability. The myocardium region, defined by the segmentation mask on the end-diastolic frame, was densely tracked through the entire cardiac cycle. Strain analysis: pixel-wise strains were calculated from the dense motion field. From the SAX slices, circumferential (Ecc) and radial (Err) strains were calculated, and from the LAX slices longitudinal (Ell) strain was calculated. Global strain was calculated by averaging over the whole heart and segmental strain was calculated following 16 AHA segments defined in landmark detection. Cardiac function analysis: LV volume and LV ejection fraction were also calculated using the myocardium segmentations.9 cardio-oncology patients and 11 patients with no cancer therapy were scanned on a 1.5T scanner (uMR 570, UIH). Scan for each patient include 9 to 12 SAX cine images to cover the whole LV, and 2-, 3- and 4-chamber LAX cine. Cine images were acquired using bSSFP sequence with the following parameters: TR/TE=2.98/1.4 ms, slice thickness = 8 mm, pixel size = 1.9x1.6 mm2, cardiac phases = 25. fastSENC was acquired on each patient at 3 SAX locations and 2-, 3- and 4-chamber LAX locations. fastSENC parameters include: TE = 0.8 ms, slice thickness = 10 mm, tagging distance = 4.9 mm, pixel size = 4x4 mm2. The fastSENC images were exported for offline analysis using Myostrain 5.1.4 (Myocardial Solutions Inc) by an experienced observer. Correlation between autoFT and fastSENC was analyzed using linear regression and Bland-Altman analyses on Ell and Ecc. Global and segmental Ell, Ecc and Err were compared between oncology and non-oncology patients.
Results
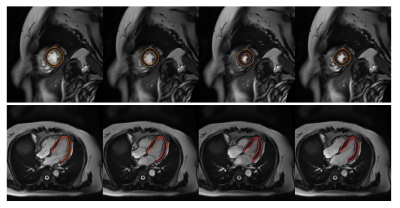
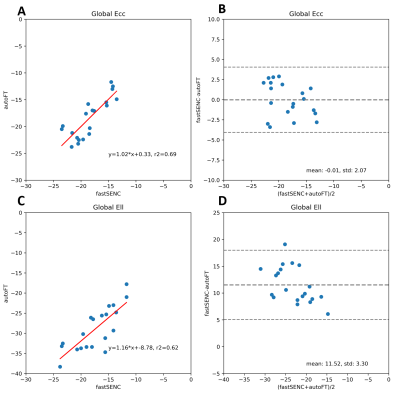
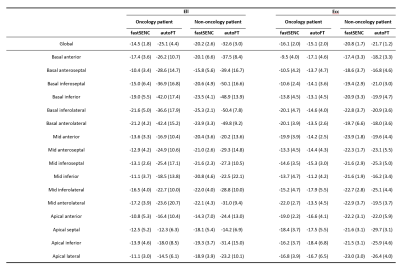
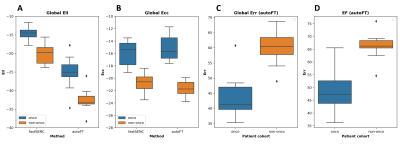
An example tracking results are shown in Fig.2. Fig.3 reveals the correlation of global Ell and Ecc of autoFT and fastSENC on all 20 patients. Global Ecc shows good correlation between autoFT and fastSENC (slope: 1.0, r2=0.7, bias=-0.0, LOA=±4.1). Global Ell shows a noticeable bias of an overestimated autoFT (slope: 1.2, r2=0.6, bias=11.52, LOA=±6.5). Fig.4 shows the 16 AHA segmental Ell, Ecc of the proposed method and fastSENC on the two patient cohorts. Fig.5 shows the distribution of global Ell, Ecc of autoFT and fastSENC on the two patient cohorts. Global Err and EF from autoFT are also shown.Discussions and Conclusions
In this study, we present a fully-automatic pipeline powered by deep learning for myocardium strain assessment through feature-tracking. The proposed pipeline realizes feature-tracking with zero human intervention and thus has no issue of observer variation. The main components, including segmentation and motion tracking leverage the recent progress in computer vision, deep learning specifically, to achieve high accuracy, robustness and computation speed, which has been demonstrated separately but not as a complete system. To the best of our knowledge, this is the first fully-automatic strain analysis system powered completely by deep learning. We show that the fully automatic FT correlates well with fastSENC for Ecc but there is a systematic bias for Ell. The bias can occur because the SENC technique calculates through-plane strains while FT calculates in-plane strains as well as the differences in spatial/temporal resolution and segment definitions. Previous studies have also shown systematic bias for Ell from FT comparing to fastSENC [13]. Note that in [13] human intervention was involved to adjust tracking results. We also show that the fully automatic FT can effectively distinguish oncology patient by strain assessment. The heterogeneous effects observed from segmental strains agrees with previous findings [3]. Future comprehensive studies will be needed to relate the value of myocardium strain assessments among oncology patients using the proposed automatic pipeline.Acknowledgements
No acknowledgement found.References
1. Victoria Delgado, Laurens F. Tops, Rutger J. Van Bommel, Frank Van Der Kley, Nina Ajmone Marsan, Robert J. Klautz, Michel I.M. Versteegh, Eduard R. Holman, Martin J. Schalij, and Jeroen J. Bax. Strain analysis inpatients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. European Heart Journal,30(24):3037–3047, 2009.
2. Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD; PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014 Feb 11;63(5):447-56. doi: 10.1016/j.jacc.2013.09.052. Epub 2013 Oct 30. Erratum in: J Am Coll Cardiol. 2014 Jul 22;64(3):335. PMID: 24184245; PMCID: PMC7195816.
3. Henning Steen, Moritz Montenbruck, Blaz Gerzak, Arne Kristian Schwarz, Sebastian Kelle, Sorin Giusca, Grigorios Korosoglou, Susan Dent, and Daniel Lenihan. Cardiotoxicity during Cancer Treatment Causes More Regional than Global Dysfunction: the PREFECT study. Journal of the American College of Cardiology,75(11):1824, 3 2020.
4. Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988 Oct;169(1):59-63. doi: 10.1148/radiology.169.1.3420283. PMID: 3420283.
5. Spottiswoode BS, Zhong X, Lorenz CH, Mayosi BM, Meintjes EM, Epstein FH. Motion-guided segmentation for cine DENSE MRI. Med Image Anal. 2009 Feb;13(1):105-15. doi: 10.1016/j.media.2008.06.016. Epub 2008 Jul 9. PMID: 18706851; PMCID: PMC2614556.
6. Osman NF, Sampath S, Atalar E, Prince JL. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med. 2001 Aug;46(2):324-34. doi: 10.1002/mrm.1195. PMID: 11477637.
7. Li Pan, Matthias Stuber, Dara L. Kraitchman, Danielle L. Fritzges, Wesley D. Gilson, and Nael F. Osman. Real-time imaging of regional myocardial function using fast-SENC. Magnetic Resonance in Medicine, 55(2):386–395,2006.
8. Karen Simonyan and Andrew Zisserman. Very Deep Convolutional Net-works for Large-Scale Image Recognition. arXiv:1409.1556 [cs.CV] 2015.
9. Olaf Ronneberger, Philipp Fischer, and Thomas Brox. U-net: Convolutional networks for biomedical image segmentation. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 9351:234–241, 2015.
10. Hanchao Yu, Xiao Chen, Humphrey Shi, Terrence Chen, Thomas S. Huang, and Shanhui Sun. Motion Pyramid Networks for Accurate and Efficient Cardiac Motion Estimation. MICCAI 2020.
11. Pingjun Chen, Xiao Chen, Eric Z. Chen, Hanchao Yu, Terrence Chen, and Shanhui Sun. Anatomy-Aware Cardiac Motion Estimation. MICCAI 2020-MLMI.
12. Hanchao Yu 2, Shanhui Sun, Haichao Yu, Xiao Chen, Honghui Shi, Thomas Huang, and Terrence Chen. FOAL: Fast Online Adaptive Learning for Cardiac Motion Estimation. Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2020.
13. Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, Steen H, Stehning C, Pieske B, Pieske-Kraigher E, Schuster A, Lapinskas T, Kelle S. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. 2020 Apr;7(2):523-532. doi: 10.1002/ehf2.12576. Epub 2019 Dec 4. PMID: 31800152; PMCID: PMC7160507.
14. Backhaus SJ, Metschies G, Billing M, Kowallick JT, Gertz RJ, Lapinskas T, Pieske B, Lotz J, Bigalke B, Kutty S, Hasenfuß G, Beerbaum P, Kelle S, Schuster A. Cardiovascular magnetic resonance imaging feature tracking: Impact of training on observer performance and reproducibility. PLoS One. 2019 Jan 25;14(1):e0210127. doi: 10.1371/journal.pone.0210127. PMID: 30682045; PMCID: PMC6347155.
Figures