2641
A comparison of spiral trajectories in a deep learning reconstruction for DENSE1Translational Data Science and Informatics, Geisinger, Danville, PA, United States, 2Medical and Health Physics, Geisinger, Danville, PA, United States, 3Heart Institute, Geisinger, Danville, PA, United States, 4Radiology, Geisinger, Danville, PA, United States
Synopsis
Displacement Encoding with Stimulated Echoes (DENSE) is a powerful technique that has found great utility in accurately measuring cardiac tissue displacement. However, DENSE remains time-consuming to acquire, particularly for 3-dimensionally encoded or higher resolution schemes, so methods to accelerate image acquisition are needed. Deep learning has shown promise to assist with a myriad of reconstruction problems, including DENSE. Here, we explore the reconstruction performance of a non-Cartesian Deep Cascade of Convolutional Neural Networks (DCCNN) when presented with undersampled data generated from multiple spiral trajectory designs and acceleration rates.
Introduction
Cine Displacement Encoding with Stimulated Echoes (DENSE) is a powerful but time-consuming technique used to obtain accurate and reproducible myocardial tissue displacement measurements.1 Efforts to speed up DENSE have included an early switch to spiral trajectories,2 reduced field of view techniques,3 as well as parallel-imaging and compressed-sensing approaches to reconstruction of undersampled data.4In a preliminary exploration using deep learning-based reconstructions for accelerated DENSE imaging, modifications to a Deep Cascade of Convolutional Neural Networks (DCCNN) reconstruction to support non-Cartesian DENSE imaging were shown to be feasible;5,6 however, only one trajectory and acceleration rate were studied in that work. Here, we sought to further investigate the performance of the reconstruction when presented with data derived from different spiral trajectory designs and at different acceleration rates.
Methods
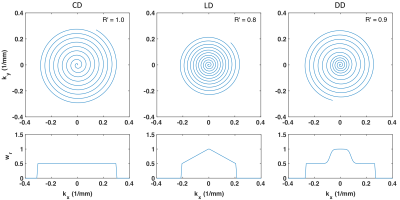
DENSE data obtained previously from 5 volunteers were used to generate train and test image sets.7 Data were originally acquired with 12-interleaf, 5000-sample constant density spiral trajectories. Pseudo raw data were generated by inverse gridding complex DENSE data using three 12-interleaf, 5000-sample spiral trajectories in which the density of spiral interleaves was varied: constant-density (CD), linear variable-density (LD), and dual-density (DD). Undersampled data were generated by setting 6, 8, 9, or 10 evenly spaced interleaves to zero, resulting in simulated acceleration rates of 2, 3, 4, and 6, respectively. The LD trajectory was designed such that the center of k-space remained fully-sampled after undersampling, and the DD trajectory was similarly designed such that the first 33% of the trajectory remained fully-sampled after undersampling (Fig. 1).A non-Cartesian 2D DCCNN reconstruction was applied for undersampled image reconstruction.5,6 Five-fold cross validation was performed, with all data from each subject reserved as the test dataset for a given fold, resulting in training sets of between 1027-1153 images, and test sets of between 186-312 images, depending on the number of slices and cardiac frames acquired per subject.
The reconstructed DENSE images were compared to the fully-sampled reference using peak SNR (PSNR). Additionally, magnitude SNR was recorded at peak systole by the mean magnitude of a region of interest (ROI) comprising the septal myocardium, divided by the standard deviation of a region of air outside the body. Phase SNR was measured as the mean phase of the peak systole septal ROI divided by the standard deviation of a liver ROI. Image quality metrics were compared between trajectories at each acceleration rate using one-way ANOVA with Tukey post hoc testing between groups. DENSEanalysis8 was used to calculate left ventricular (LV) strains from each trajectory/acceleration rate combination, and the differences in the resulting myocardial radial, circumferential, and longitudinal strain measurements generated by the reconstruction compared to the fully-sampled reference were evaluated with one-way ANOVA with Tukey post hoc testing (p < 0.05 considered significant).
Results
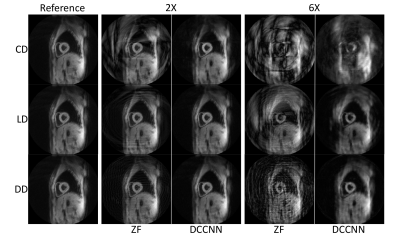
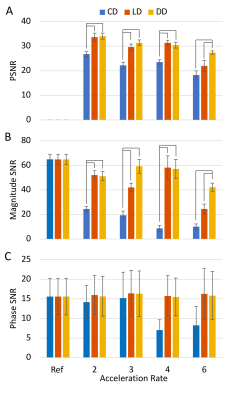
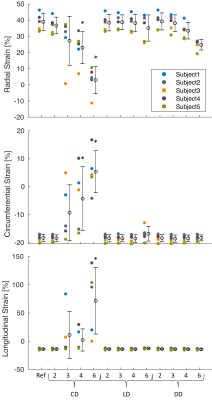
Magnitude images from the peak systolic phase of one subject are shown in Figure 2, demonstrating typical reconstruction performance for each trajectory type at two acceleration rates. Both variable density (VD; i.e., LD and DD) trajectories’ PSNR generally outperformed the CD trajectory across acceleration rates. Similar trends were observed in the myocardial magnitude measurements, with both VD trajectories exhibiting higher SNR than the CD design. Phase SNR was less affected by trajectory and acceleration rate (Fig. 3).Significant differences from the reference radial, circumferential, and longitudinal strains were measured for the CD trajectory at 6X acceleration, and for radial and circumferential at 4X (Fig. 4). No other differences were observed, although the DD trajectory trended toward underestimation of radial strains at high acceleration rates (p>0.05).
Discussion
In this study, the performance of a deep learning-based reconstruction for retrospectively undersampled spiral DENSE data was assessed for three spiral trajectory designs. We demonstrated that VD trajectories generally outperformed a CD design across all acceleration rates, and furthermore that among VD trajectories, an LD design resulted in the smallest strain bias, although these differences were not significant. As deep learning becomes more ubiquitous in MR image reconstructions – outside of the limited DENSE application described here – these results demonstrate that the proper pairing of reconstruction and acquisition design must be carefully considered.Limitations to this study are as follows: (1) Only one trajectory design from each of the VD trajectory types was investigated. (2) All analyses were performed on retrospectively undersampled accelerated data. (3) A limited number of independent patients were included for training. (4) All segmentations were performed on fully-sampled reference data and copied to their corresponding undersampled derivatives. (5) A broad question remains as to the generalizability of deep-learning-based reconstructions. All exams included in this study were from a single scanner and research protocol with the same, tightly-controlled imaging parameters. The performance of these methods across a range of typical imaging conditions needs to be assessed.
Conclusion
We have assessed a deep learning approach for reconstructing undersampled complex-valued spiral data, such as that used in DENSE. The reconstruction was presented with undersampled data from three trajectory designs, with strain measurements from variable-density designs ultimately most similar to the fully-sampled reference data. Future implementations of deep learning for non-Cartesian MR reconstruction problems should carefully consider trajectory as part of the overall image acquisition-to-reconstruction pipeline.Acknowledgements
No acknowledgement found.References
1. Kim, D.; Gilson, W. D.; Kramer, C. M.; Epstein, F. H. Myocardial Tissue Tracking with Two-Dimensional Cine Displacement-Encoded MR Imaging: Development and Initial Evaluation. Radiology 2004, 230, 862–871.
2. Zhong, X.; Spottiswoode, B. S.; Meyer, C. H.; Kramer, C. M.; Epstein, F. H. Imaging Three-Dimensional Myocardial Mechanics Using Navigator-Gated Volumetric Spiral Cine DENSE MRI. Magnetic resonance in medicine 2010, 64, 1089–97.
3. Scott, A. D.; Tayal, U.; Nielles-Vallespin, S.; Ferreira, P.; Zhong, X.; Epstein, F. H.; Prasad, S. K.; Firmin, D. Accelerating Cine DENSE Using a Zonal Excitation. Journal of Cardiovascular Magnetic Resonance 2016, 18.
4. Chen, X.; Yang, Y.; Cai, X.; Auger, D. A.; Meyer, C. H.; Salerno, M.; Epstein, F. H. Accelerated Two-Dimensional Cine DENSE Cardiovascular Magnetic Resonance Using Compressed Sensing and Parallel Imaging. Journal of Cardiovascular Magnetic Resonance 2016, 18, 38.
5. Schlemper, J.; Caballero, J.; Hajnal, J. V; Price, A. N.; Rueckert, D. A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction. IEEE Transactions on Medical Imaging 2018, 37, 491–503.
6. Fielden, S. W.; Carruth, E. D.; Nevius, C. D.; Fornwalt, B. K.; Haggerty, C. M. Deep learning for undersampled spiral DENSE reconstruction. In International Society of Magnetic Resonance in Medicine; 2020; p. 3632.
7. Suever, J. D.; Wehner, G. J.; Jing, L.; Powell, D. K.; Hamlet, S. M.; Grabau, J. D.; Mojsejenko, D.; Andres, K. N.; Haggerty, C. M.; Fornwalt, B. K. Right Ventricular Strain, Torsion, and Dyssynchrony in Healthy Subjects Using 3D Spiral Cine DENSE Magnetic Resonance Imaging. IEEE transactions on medical imaging 2017, 36, 1076–1085, PMCID: PMC5711416.
8. Spottiswoode, B. S.; Zhong, X.; Hess, a T.; Kramer, C. M.; Meintjes, E. M.; Mayosi, B. M.; Epstein, F. H. Tracking Myocardial Motion from Cine DENSE Images Using Spatiotemporal Phase Unwrapping and Temporal Fitting. IEEE transactions on medical imaging 2007, 26, 15–30.
Figures