2635
Automated Vessel Segmentation for 2D Phase Contrast MR Using Deep Learning1Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Cleveland, OH, United States, 2Siemens Healthcare, Erlangen, Germany, 3Siemens Medical Solutions USA, Inc, Princeton, NJ, United States, 4Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 5Internal Medicine, The Ohio State University, Columbus, OH, United States, 6Davis Heart & Lung Research Institute, The Ohio State University, Columbus, OH, United States, 7Radiology, The Ohio State University, Columbus, OH, United States
Synopsis
Phase-contrast (PC) MRI is used to evaluate blood hemodynamics; however, it can be time consuming to process PC-MR data. In this work, we developed a fully automated segmentation algorithm for PC MR images using deep learning (DL). Automated segmentation of aorta and main pulmonary artery from PC MRI scans can be successfully achieved using the DL model.
Introduction
Phase-contrast (PC) MRI is a non-invasive technique that is commonly used clinically to evaluate blood hemodynamics1; however, it can be time consuming to process PC-MR data as multiple PC acquisitions are normally performed in different 2D anatomical orientations in each patient, and manual segmentation is needed to analyze 20-30 frames from each PC scan. We developed a fully automated segmentation algorithm for PC MR images using deep learning (DL).Methods
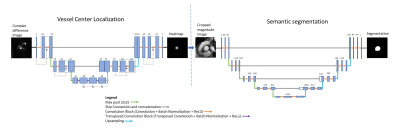
The segmentation model includes two convolutional neural networks (CNN) (Fig. 1). The vessel center is first localized using a 2D U-net model with 3 encoder-decoder blocks2 to regress heatmaps directly from input images3 . The model is trained using the magnitude of complex difference images in the systolic phase as input and Gaussian heatmap as output. Since only pixels with flow are bright in complex difference images in the systolic phase, four such images from each series were calculated and used as the input. A Gaussian heatmap was calculated based on $$$G_{\sigma}=e^{-(\frac{(x-x_{0})^{2}}{2\sigma^{2}}+\frac{(y-y_{0})^{2}}{2\sigma^{2}})}$$$ where x0 and y0 were the coordinates of the vessel center and σ defines the peak width of Gaussian function in the heatmap. σ was set to 16 and was reduced gradually $$$\sigma=0.99^{epochs}*16$$$ in the first 100 epochs and then was fixed to 5 in the next 100 epochs. The vessel center was calculated as the maximum location of the output heatmap, then the magnitude images were cropped to 60 x 60 pixels centered on the vessel and interpolated to 256 x 256. A second 2D U-net model with 5 encoder-decoder blocks was trained to segment out the vessel using cropped magnitude images as input and corresponding ground-truth segmentation as output. The two CNNs were built in Python using DL framework PyTorch.For training of the segmentation model, we used a total of 360 2D PC-MR series (7219 distinct images) acquired in the aorta (AO) (n = 251) and main pulmonary artery (MPA) (n = 109) from 23 normal volunteers scanned on a 3T scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany) as well as 240 patients referred for clinical cardiac MR scans on 1.5T scanners (n=177 on MAGNETOM Avanto and n=63 on MAGNETOM Sola, Siemens Healthcare). Manual segmentation by Segment (Medviso AB, Lund, Sweden) was used as the ground truth. For testing, we used 32 additional 2D PC-MR series (23 in AO and 9 in MPA) from cardiac MR patients acquired on a 1.5T scanner (MAGNETOM Sola) to compare the automated vessel segmentation to the manual segmentation and the flow quantification results.
Results
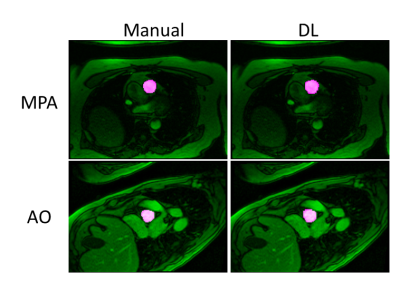
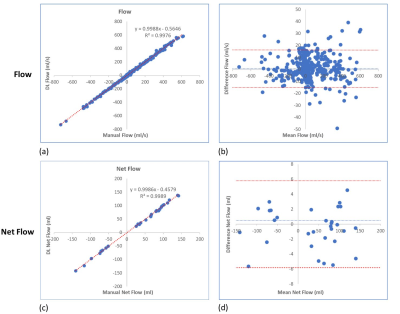
The average processing time for automatic vessel segmentation was 1.88 ± 0.14 seconds for each data series on a personal computer with 16GB of system memory and a graphics processing unit with 8 GB of video memory (Quadro M2000M, NVIDIA, Santa Clara, CA, USA). Automated vessel segmentation successfully identified and segment out the correct vessel in 31/32 PC-MR cases without any user interaction. In one AO PC-MR case, auto-segmentation detected and segmented the incorrect vessel in 6 frames in the diastolic phase and hence this case was excluded from the subsequent analysis. Table 1 shows the dice scores obtained after DL segmentation on testing datasets for AO and MPA. Figure 2 shows the representative example of vessel contouring performed by manual and DL segmentation. Figure 3 shows that there was a strong correlation between flow of each cardiac phase from DL and flow from manual segmentation (R2 > 0.99), without a statistical difference (p = 0.13). Similar results were observed for net flow (see Figure 3).Conclusions
We developed a DL model for fully automated vessel segmentation and flow quantification in 2D PC-MRI images, alleviating the cumbersome and time-intensive process to manually draw vessel contours on individual phase contrast images. Automated segmentation of AO and MPA from PC MRI scans can be successfully achieved using the DL model in most cases. Future work will extend this approach for segmentation of other types of blood vessels, such as left/right pulmonary arteries and valves, to make it more broadly applicable to any PC-images. Also, we plan to integrate this approach on the scanner, so the operator can view the flow quantification results immediately and use this information to guide additional acquisitions if abnormalities are detected.Acknowledgements
No acknowledgement found.References
1. Joachim Lotz et al., Cardiovascular Flow Measurement with Phase-Contrast MR Imaging: Basic Facts and Implementation, RadioGraphics, Vol.22, No. 3
2. Olaf Ronneberger et al., U-Net: Convolutional networks for biomedical image segmentation. Medical image computing and computer-assisted intervention – MICCAI 2015: Springer International Publishing; 2015. p. 234–41.
3. Christian Payer et al., Integrating spatial configuration into heatmap regression based CNNs for landmark localization, Medical Image Analysis 54 (2019) 207–219
Figures