2617
High Efficient Reconstruction Method for IVIM Imaging Based on Deep Neural Network and Synthetic Training Data and its Application in IVIM-DKI1Department of Electronic Science, Xiamen University, Xiamen, Fujian, China, 2Department of Radiology, First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China
Synopsis
Intravoxel incoherent motion (IVIM) imaging is a non-invasive MR perfusion imaging that could prevent patients from the harm of exogenous reagent. Previous studies proved that the least square and Bayesian approaches are so far the best algorithms in IVIM fitting. However, they still suffer from time-consuming and high noise level. We proposed a deep neural network-based reconstruction method with synthetic training data for IVIM imaging and extended it to hybrid IVIM-DKI (diffusion kurtosis imaging) model fitting. Experimental results show that our method owns prominent performance in both image quality and accuracy of fitting results with a remarkably short reconstruction time.
Introduction
IVIM biexponential model takes diffusion and perfusion effects of water molecule into consideration simultaneously,1 which makes it possible to obtain patient’s microcirculation network perfusion information without injection of contrast agent. Conventional IVIM fitting methods2,3 are in the mode of pixel by pixel and thus take long reconstruction time. Moreover, the independence of each pixel fitting process easily leads to outliers and distinct graininess. Considering the influence of surrounding pixels, we introduced U-Net with large receptive field into IVIM fitting to eliminate excessive outliers and achieve image smoothing.Method
In vivo DWI images were collected using ten b values for IVIM and thirteen b values for IVIM-DKI on 3T SIEMENS Skyra scanners. As an adequate amount of real training samples is hardly obtainable and ideal training label from in vivo data is also unavailable, synthetic training samples were utilized for network training. In the thought of validating the robustness of our method, we extended our method into IVIM-DKI model fitting. Specific IVIM and hybrid IVIM-DKI model are as follows:$$S(b)=S_{0}((1-f)e^{-bD}+fe^{-bD^{*}}) $$
$$S(b)=S_{0}((1-f)e^{-bD+\frac{1}{6}(bD)^{2}K}+fe^{-bD^{*}})$$
where D is the pure diffusion coefficient, f is the perfusion fraction, D* is the pseudo-diffusion coefficient, and K is the diffusion kurtosis coefficient; S0 and S(b) are the signals obtained without and with diffusion encoding gradient, respectively.
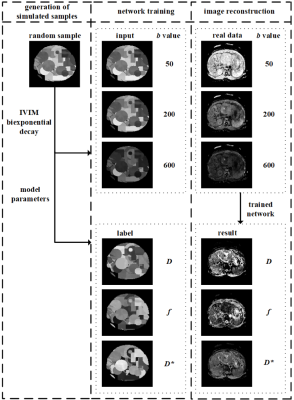
Figure 1 shows the flow chart of the proposed method. There are roughly three parts in the whole process:
Training samples generation: (1) The D, f and D* (D, f, D* and K for IVIM-DKI model) parametric maps in certain value ranges with suited amount of geometric shapes (circle, rectangle, triangle and ring were involved in our method) were randomly generated. (2) The values of each pixel in the D, f and D* parametric maps were used to calculate corresponding signal intensity value under different b value according to IVIM model. (3) A set of D, f and D* parametric maps together with the synthetic DWI images form a training sample. The above steps were repeated to acquire sufficient samples for network training.
Network training: The synthetic DWI images were used as the network input, and the corresponding randomly generated D, f and D* parametric maps were employed as the labels.
Network prediction: In vivo DWI images were fed into the trained network, then related IVIM parameters were estimated and parametric maps were reconstructed.
Results
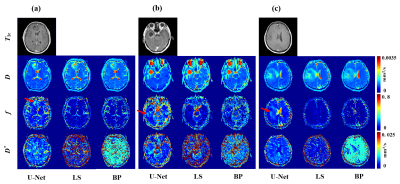
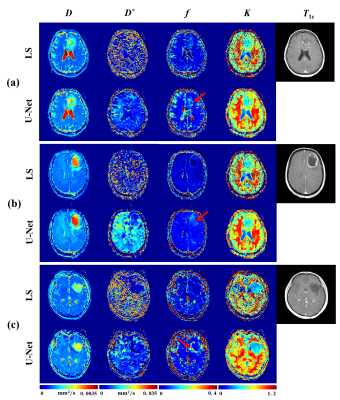
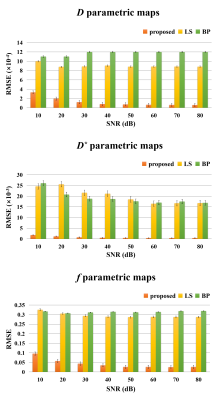
Figure 2 illustrates fitting results of three types of glioma named oligodendroglioma (Fig. 2a), IDH wild-type astrocytoma (Fig. 2b), and IDH-mutated astrocytoma (Fig. 2c) using IVIM model. Postcontrast T1 weighted images are given to display approximate lesion appearance of each patient. Compared to least square2 and Bayesian3 approaches, our U-Net based reconstruction method produces clearer and better parametric maps without losing lesion details. Other than that, more perfusion information (the areas indicated by red arrows) are obviously contained in our f parametric maps, which means our method get more accurate lesion composition. Figure 3 shows fitting results of IVIM-DKI model on exactly the same cerebral tumor types as Figure 2. Similarly, noise is reduced greatly on parametric maps derived from our proposed method, while least square curve-fitting4 fabricates severe graininess. Furthermore, we evaluated the veracity of different algorithms using synthetic DWI data under different signal-to-noise (SNR) in IVIM fitting. Root mean square error (RMSE) was employed as assessment criterion and calculation results in Figure 4 suggest an apparently high accuracy of our method since U-Net is associated with the lowest RMSE for all 3 parameters. The fitting time is 5-7 minutes for the least square approach, 20-30 minutes for the Bayesian approach, and several seconds for our proposed method. That is, our method takes orders of magnitude shorter time than classical ones. In brief, the proposed IVIM reconstruction method is better, faster and stronger than previous common algorithms.Discussion
We put forward a training sample generation method based on theoretical model for neural network to surmount the shortage of ground truth. The reconstructed results for the two models studied herein reveal its applicability. According to our experiments, the quality of parametric maps obtained from the proposed method moderately depends on the selection of preset parameter value range. An understandable explanation is the preset parameter value range could be regarded as prior knowledge for network learning. The degree of consistency of parameter value ranges between synthetic samples and real experimental data could have certain impact on neural network outputs.Conclusion
The proposed U-Net based IVIM reconstruction method shows better results than least square and Bayesian algorithm. It achieves more precise quantitative perfusion results and far less noisy parametric maps in an efficient way, showing significant potential in clinical applications as an auxiliary diagnosis tool.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers 11775184 and 81671674.References
1. Lebihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions-application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-407.
2. Jalnefjord O, Montelius M, Starck G, et al. Optimization of b-value schemes for estimation of the diffusion coefficient and the perfusion fraction with segmented intravoxel incoherent motion model fitting. Magn. Reson. Med. 2019;82:1541-1552.
3. Gustafsson O, Montelius M, Starck G, et al. Impact of prior distributions and central tendency measures on Bayesian intravoxel incoherent motion model fitting. Magn. Reson. Med. 2018;79:1674-1683.
4. Wen CW, Shun CY, Ya FC, et al. Simultaneous assessment of cerebral blood volume and diffusion heterogeneity using hybrid IVIM and DK MR imaging: initial experience with brain tumors. Eur. Radiol. 2017;27:306-314.
Figures