2615
Simultaneous T1- and T2-weighted imaging using RF phase modulated gradient echo imaging1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 4Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
A novel method for simultaneous T1 and T2-weighted imaging using RF phase-modulated GRE with small RF phase increments is presented. Configuration theory approach was used to derive an equation for the steady-state GRE signal. The equation reveals that small RF phase increments provide separable T1 and T2-weighted contrast in the real and imaginary components of the signal. Simulation and phantom studies were performed for quantitative analysis of the proposed method. Brain in-vivo imaging was included to show clinical feasibility. The results suggested the proposed method enables faster imaging compared to conventional FSE imaging.
INTRODUCTION
T1 and T2-weighted (T1w, T2w) imaging, typically performed using fast spin-echo (FSE)-based methods, are widely used for clinical MR examinations. Despite recent progress of fast imaging technique(1)(2), there is still a need to shorten scan times. In addition, FSE-based methods are generally limited to 2D acquisitions for most clinical applications.Recently, a T2 mapping method using RF phase modulated gradient echo (GRE) imaging, with small RF phase increments, was proposed(3). In this previous work, we demonstrated the ability to encode T2 information into the GRE signal phase.
In the current work, we now propose a novel method for simultaneous T1w and T2w imaging, which is achieved by encoding contrast information into the real and imaginary components of a signal, using GRE imaging with RF phase modulation.
THEORY
In this work, we use Sobol's approach to explain the steady-state characteristics of the RF phase-modulated GRE signal(4). The RF phase modulation was performed by incrementing the transmit RF phase ($$$\phi$$$) quadratically such that $$$\phi (n) = \phi (n-1) + n \theta$$$, where $$$theta$$$ is the RF phase increment. The signal with RF pulse with flip angle (FA) of α, RF phase of $$$\phi$$$, and a repetition time of TR can be expressed as$$Re(S) = \beta \eta e^{-\frac{TR}{T2}}$$
$$Im(S) = \beta [\eta^2 - \epsilon (e^{-\frac{TR}{T2}}-\epsilon)]$$
with
$$\beta = \frac{ (1-e^{-\frac{TR}{T1}}) M_0 sin \alpha}{ (e^{-\frac{TR}{T2}} - \epsilon) [ e^{-\frac{TR}{T2}} (cos \alpha - e^{-\frac{TR}{T1}}) + \epsilon (1 - e^{-\frac{TR}{T1}} cos \alpha) ] - \eta^2 (1 - e^{-\frac{TR}{T1}} cos \alpha) }$$
where M0 is the proton density, and $$$\epsilon$$$ and $$$\eta$$$ are real coefficients, determined by recursive calculation depending on T1, T2, TR, α, and θ (4). The contrast of the GRE is sensitive to θ while it also depends on TR and α.
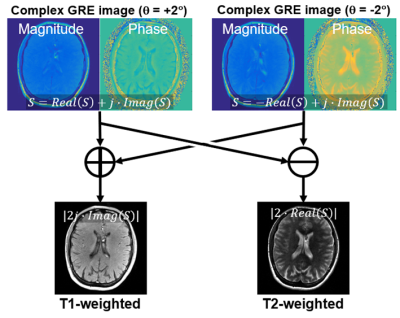
In general, the acquired signal includes a background phase. Two-pass imaging was adopted to remove this, achieved by using acquisitions with positive and negative polarities of RF phase increments, as explained in Fig. 1.
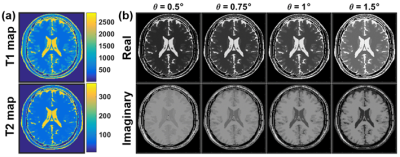
To show the contrast dependency on θ, a simulation study was performed using a numerical brain phantom, downloaded from the MRiLab project(5). The GRE signal of the brain phantom was calculated using Eq. 1 and 2 with of 0.5, 0.75, 1.0, and 1.5°.
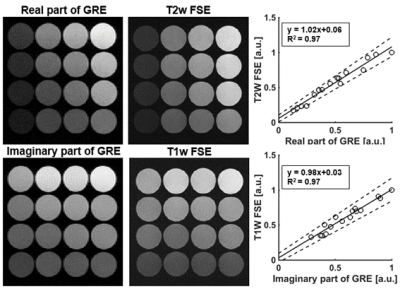
A phantom consisting of 16 vials with different T1 and T2 values was imaged using the proposed method. Conventional T1w and T2w FSE images were also obtained as references. The intensity of the acquired signal was measured and compared between the proposed method and FSE. The intensities were normalized using the maximum intensity of the images.
We evaluated the feasibility of simultaneous T1w and T2w imaging of the brain comparing the proposed method with FSE. Healthy volunteers were recruited from an Institutional Review Board (IRB) approved database of healthy volunteers.
RESULTS
As shown in Figure 2, simulations demonstrated that T1w and T2w contrast can be encoded into the GRE signal's real and imaginary components. Figure 2a shows quantitative T1 and T2 maps of the numerical phantom used in this study. Figure 2b demonstrates that that T1 weighting increases as θ decreases while stronger T2 weighting can be obtained as θ increases.Figure 3 shows T1w and T2w images of the phantom acquired using the proposed method and with FSE. The signal intensities of the real and imaginary components of the proposed method agreed well with T2w (R2 = 0.97) and T1w (R2 = 0.97) FSE signals as shown in the plot in Figure 3.
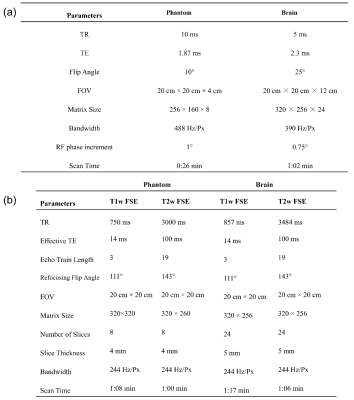
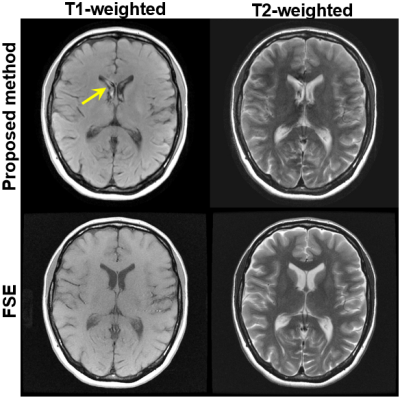
Example images of the brain using the proposed method compared with FSE imaging are shown in Figure 4. Typical T1w and T2w contrasts of the brain were observed using the proposed method, although there is a slight mismatch of the contrast of gray-white matter in the proposed method compared to FSE imaging. Also, possible motion-induced artifacts were seen in the CSF (arrow). Acquisition for the proposed method was 1:02 min, while FSE imaging for T1w and T2w required a total of 2:23 min. Imaging parameters for phantom and volunteer experiments are shown in Tab. 1.
DISCUSSION
In this work, we have proposed and demonstrated the feasibility of a novel RF phase modulated 3D GRE imaging method to obtain simultaneous T1w and T2w images. Our results demonstrated that the proposed method enables fast acquisition with T1w and T2w contrast compared to conventional FSE imaging.The proposed method could be used as an alternative to conventional T1w and T2w imaging. Several imaging techniques using GRE imaging with a small RF phase increment have been proposed to obtain various contrast mechanisms(6) and quantitative maps(7), which utilize the magnitude of the acquired signal. On the other hand, the proposed method provides T1w and T2w contrasts from the real and imaginary components simultaneously, resulting in reducing scan time.
There are limitations of the proposed method. Fat suppression was not included in this study. It should be straightforward to combine chemical shift encoded water-fat separation strategies to facilitate fat suppression. Other potential limitations such as motion and short T2* should be investigated for clinical use.
CONCLUSION
We successfully developed and demonstrated the feasibility of a novel RF phase modulated 3D GRE imaging method for rapid simultaneous T1w and T2w imaging.Acknowledgements
We wish to acknowledge support from the UW-Madison Department of Radiology, UW Institute for Clinical and Translational Research, and the Clinical and Translational Science Award of the NCATS/NIH. Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 1999;42(5):952-962.
2. Lustig M, Donoho D, Pauly JMJMRiMAOJotISfMRiM. Sparse MRI: The application of compressed sensing for rapid MR imaging. 2007;58(6):1182-1195.
3. Wang X, Hernando D, Reeder SB. Phase‐based T2 mapping with gradient echo imaging. Magnetic Resonance in Medicine 2019.
4. Sobol WT, Gauntt DM. On the stationary states in gradient echo imaging. Journal of Magnetic Resonance Imaging 1996;6(2):384-398.
5. Liu F, Velikina JV, Block WF, Kijowski R, Samsonov AA. Fast realistic MRI simulations based on generalized multi-pool exchange tissue model. Ieee T Med Imaging 2016;36(2):527-537.
6. Ganter C. Steady state of gradient echo sequences with radiofrequency phase cycling: Analytical solution, contrast enhancement with partial spoiling. Magnetic Resonance in Medicine 2006;55(1):98-107.
7. Bieri O, Scheffler K, Welsch GH, Trattnig S, Mamisch TC, Ganter C. Quantitative Mapping of T-2 Using Partial Spoiling. Magnetic Resonance in Medicine 2011;66(2):410-418.
Figures