2614
Simultaneous multi-slice 3D Spatiotemporal Encoding (SPEN) Imaging: Emulation study1Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 2Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of, 3Siemens Healthineers, Seoul, Korea, Republic of, 4Biomedical Institute for Convergence at SKKU, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Acceleration imaging techniques such as parallel imaging and simultaneous multi-slice (SMS) imaging have been developed to achieve shorter acquisition time and larger FOV and incorporated into various imaging techniques including ultrafast imaging techniques such as echo-planar imaging (EPI). Here, we propose a new way to accelerate 3D spatiotemporal encoding (SPEN) imaging by combining SMS with controlled aliasing for parallel imaging results in higher acceleration (CAIPIRINHA) and split slice generalized auto-calibrating partially parallel acquisitions (Split Slice-GRAPPA).
Introduction
Acceleration imaging techniques such as parallel imaging and simultaneous multi-slice (SMS) imaging have been developed to achieve shorter acquisition time and larger FOV and incorporated into various imaging techniques including ultrafast imaging techniques such as echo-planar imaging (EPI). Parallel imaging and SMS imaging techniques were also used to accelerate spatiotemporal encoding (SPEN) imaging which is non-conventional type of ultrafast scanning method using non-Fourier encoding and have high tolerance to B0 inhomogeneity effects including magnetic susceptibility artifacts. For example, Schmidt et al. recently proposed a parallel SPEN imaging method that combines multiband frequency-swept pulse with sensitivity encoding (SENSE) in the super-resolved (SR) SPEN image reconstruction1. Despite being a good study, they discussed that the SR image reconstruction combined with SENSE was imperfect showing image distortion at the seam regions. In this study, we propose a way to accelerate 3D SPEN imaging by combining SMS with controlled aliasing for parallel imaging results in higher acceleration (CAIPIRINHA)2 and split slice generalized auto-calibrating partially parallel acquisitions (Split Slice-GRAPPA)3. The proposed method was demonstrated in the emulated data which was created by combining two 3D SPEN imaging data sets obtained with different slab positions, i.e., using two single-band chirp pulses with different frequency bands.Methods
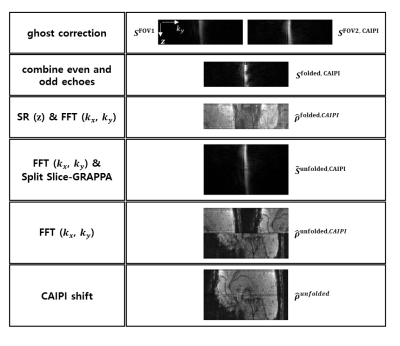
Pulse sequence: The proposed method was tested using a 3D ultrafast gradient-echo-based SPEN imaging sequence, called ERASE (Equal-TE Rapid Acquisition with Sequential Excitation)4, which provides a constant TE across slices and high tolerance to B0 inhomogeneity. Slab selection was conducted in the SPEN direction (Fig. 1a).Emulating SMS-ERASE with CAIPI: Two linearly frequency-swept chirp pulses of single band were used to acquire two ERASE imaging data sets under-sampled in half having a different frequency band (or different slab position), instead of using a multiband pulse. To emulate phase modulation in the CAIPI approach, the k-spaces of the acquired two data sets were interleaved line by line to make a full k-space after an additional phase of π radian was added to one data set in the secondary phase-encoding direction, not the SPEN direction (Fig. 1b).
Image Reconstruction with Split Slice GRAPPA: Images were reconstructed from the full k-space emulated above using the SR image reconstruction algorithm by Chen et al.5 with the phase correction of even and odd lines in k-space, which present the aliased images which emulate the SMS-ERASE with CAIPI (Fig. 1c). After the SR reconstruction, Split Slice-GRAPPA was applied to unfold the aliasing of the SMS-ERASE with CAIPI. The detailed reconstruction procedure is summarized in Fig. 2.
Experiments: All experiments were performed at 3T (Prisma, Siemens) using a 64-channel head/neck coil. Scan parameters of ERASE imaging were: FOV = 216×216 mm2, matrix size = 108×108, number of slices = 32, slice thickness = 1 mm, TR = 60 ms, TE = 28 ms, pulse time-bandwidth product (= Te×BW) = 93, pulse length (Te) = 24.68 ms, and multiband acceleration factor = 2.
Result & Discussion
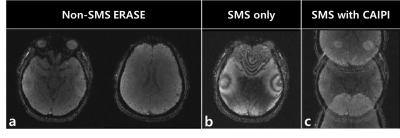
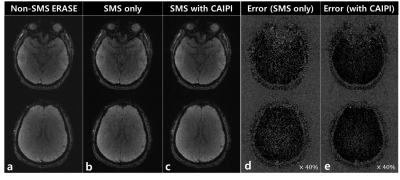
Figure 3 shows the original non-SMS ERASE images for reference (a), a fully aliased SMS-ERASE image with no CAIPI (b), and a partially aliased SMS-ERASE image with CAIPI (c). Figure 4 shows that SMS-ERASE images can be unfolded by the SR reconstruction combined with Split Slice-GRAPPA. In addition, the SMS-ERASE images with CAIPI (c and e) showed better image quality, i.e., better delineation of internal structures with less noise, than the SMS-SENSE images with no CAIPI (b and d). The error images in d and e were obtained by subtracting b and c from a, respectively. Further studies are needed to confirm this result actually when using a multiband frequency-swept pulse and in higher acceleration.Conclusion
It was demonstrated in emulation data that SMS imaging could be integrated into 3D SPEN imaging (e.g., ERASE) using Split Slice-GRAPPA and shows better performance with CAIPIRINHA.Acknowledgements
This work was supported by NRF-2019M3C7A1031993.References
[1] Schmidt R, Baishya B, Ben-Eliezer N, Seginer A, Frydman L. Super-resolved parallel MRI by spatiotemporal encoding. Magn. Reson. Imag. 2014;32:60–70.
[2] Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi‐slice imaging. Magn. Reson. Med. 2005;53(3):684-691.
[3] Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice Leakage Artifact Reduction Technique for Simultaneous Multislice Acquisitions. Magn. Reson. Med. 2014;72:93-102.
[4] Ryu JK, Jung WB, Yu J, Son JP, Lee SK, Kim SG, Park JY. An equal-TE ultrafast 3D gradient-echo imaging method with high tolerance to magnetic susceptibility artifacts: Application to BOLD functional MRI. Magn. Reson. Med. 2020;DOI:10.1002/mrm.28564.
[5] Chen Y, Li J, Qu XB, Chen L, Cai CB, Cai SH, Zhong JH, Chen Z. Partial Fourier transform reconstruction for single-shot MRI with linear frequency-swept excitation. Magn. Reson. Med. 2013;69:1326-1336.
Figures