2607
Accelerating 3D variable-flip-angle T1 mapping: a prospective study based on SUPER-CAIPIRINHA1Institute of Medical Imaging Technology, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2United Imaging Healthcare Co., Ltd, Shanghai, China
Synopsis
Three-dimensional variable-flip-angle (VFA) T1 mapping is a valuable T1 quantification method subject to a long scan time due to acquisition of multiple 3D images. SUPER (Shift Undersampling improves Parametric-mapping Efficiency and Resolution) is a recently developed method providing fast blockwise reconstruction for accelerated parametric mapping. Here we develop a combination of SUPER and 3D CAIPIRINHA to achieve 5-fold acceleration with 5 flip angles, validated with both retrospective and prospective reconstruction. The results suggest that the proposed method is highly accurate for accelerating 3D VFA T1 mapping, reducing the whole-brain scan time from 6 to 1.5 minutes.
Introduction
Three-dimensional variable-flip-angle (VFA) T1 mapping is a rapid T1 quantification method1-3 with valuable clinical applications for brain4-6 and liver7-10 imaging. However, long scan time is required to obtain multiple 3D images with different flip angles. Parallel imaging with CAIPIRINHA11 is among the most common acceleration techniques for accelerating 3D imaging. SUPER (Shift Undersampling improves Parametric-mapping Efficiency and Resolution)12 is a recently developed model-based method, which provides fast blockwise reconstruction for accelerated parametric mapping. We have previously developed SUPER-SENSE—a combination of SUPER and 2D parallel imaging—for accelerating 3D VFA T1 mapping. The method was validated with retrospective data only. Here we develop a combination of SUPER and CAIPIRINHA to achieve higher acceleration rates, and we validate the novel technique with both retrospective and prospective data.Methods
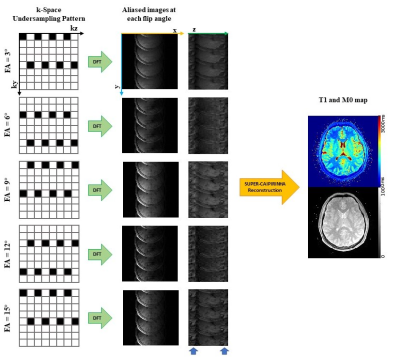
The combination of SUPER and CAIPIRINHA, herein named SUPER-CAIPIRINHA, leverages both temporal and volumetric coil sensitivity encoding to achieve a 5-fold acceleration rate within a scan of 5 flip angles. A 5-fold SUPER-CAIPIRINHA acceleration pipeline for VFA T1 mapping is illustrated in Figure 1. In the first step, aliased images were generated by performing Discrete Fourier Transform to k-space, which was undersampled by an interleaved CAIPIRINHA sampling pattern. Of the 5-fold aliasing, 4-fold is along the phase encoding direction and the other 1.25-fold is along the slice-encoding direction. To reconstruct the unaliased parameter maps from the aliased images, the blockwise curve-fitting cost function formulated based on the SUPER framework12 is minimized. Specifically, the following cost function is minimized for each block:∑m∑l |yml - wlr HSm∅l(x)|2
where yml represents value of the signal at the lth flip angle and the mth coil where l=0, 1, ..., L-1, and m=0, 1, ..., M-1, ∅l(x) represents the model evaluated at the lth flip angle defined by ∅l([M0,T1]T)=(M0 (1 - e-TR/T1 ) sinαl ) / (1 - e-TR/T1 cosαl) where x=[M0,T1]T is the parameters to estimate, TR is the repetition time, and αl is the lth flip angle, Sm represents the coil sensitivity of the mth coil, wlr=1/R·e(2πιlr/Ry) represents the modulating vector for each voxel in the block where r=0,1, ..., 7, R is the total acceleration rate, and Ry is the ky acceleration rate. We used Levenberg-Marquardt for minimizing the above cost function.
Six healthy subjects (age 23±1, 3 male) were imaged in a 3T scanner (uMR790, Shanghai United Imaging Healthcare, Shanghai, China) after providing written informed consent. 3D T1-weighted images were obtained using a 3D FLASH sequence with 24-channel head coil (17 used in acquisition). FOV was 300mm×300mm×150mm, covering the entire cerebrum and image size was 192×192×30. Five flip angles were used namely 3°, 6°, 9°, 12°, and 15°. Other sequence parameters were slice-thickness/TR/TE/bandwidth =5mm/8ms/1.98ms/400Hz/Pixel. k-Space was retrospectively undersampled based on SUPER-SENSE(R=4) and SUPER-CAIPIRINHA(R=5). Retrospective data and prospective data were obtained and reconstructed separately. Gray matter and white matter were segmented based on the non-acceleration T1 map using a thresholding window of [1080 ms, 1580ms] and [580 ms, 1080 ms], respectively. Averaged gray matter and white matter T1 was compared between non-acceleration, SUPER-SENSE, and SUPER-CAIPIRINHA.
Results
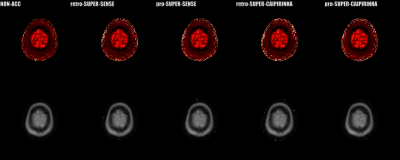
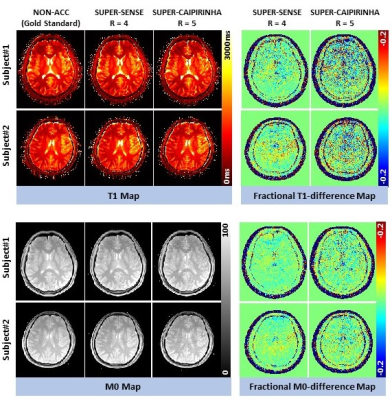
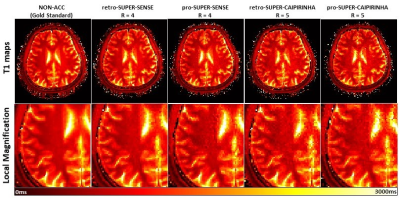
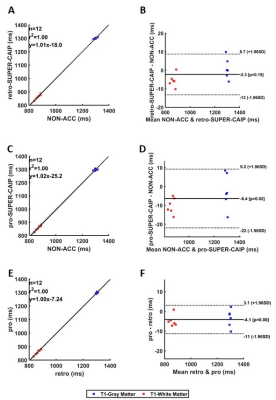
Figure 2 shows the reconstructed 3D T1/M0 maps of the entire cerebral cortex for 1 healthy subject. Image quality was similar between non-acceleration, SUPER-SENSE, and SUPER-CAIPIRINHA and between retrospective and prospective reconstruction. Figure 3 shows retrospectively accelerated T1/M0 maps, fractional T1 difference maps and fractional M0 difference maps in the central slice of 2 subjects. There was visible noise amplification due to acceleration and SUPER-CAIPIRIRINHA was slightly noisier than SUPER-SENSE. Fine details of each map were well-preserved for all methods and both subjects. Figure 4 shows the comparison of retrospective and prospective acceleration in one subject. The quality of the prospective data is consistent with that of the retrospective data, despite slightly increased noise. Figure 5 shows the results of correlation analysis and Bland-Altman analysis for evaluating the consistency of ROI-averaged T1 quantification between non-acceleration, retro-SUPER-CAIPIRINHA, and pro-SUPER-CAIPIRINHA. The difference of both gray-matter and white-matter T1 between any pair of the 3 methods was not greater than 7ms, which is less than 1% of the average T1 of gray matter (1300ms, from non-acceleration) and white matter (868ms, from non-acceleration). SUPER-SENSE and SUPER-CAIPIRINHA reduced the scan time from 6:11 minutes to 2:09 minutes and 1:29 minutes, respectively.Conclusions
In this work, we demonstrated a novel method to accelerate 3D VFA T1 mapping with a 5-fold acceleration rate by combining SUPER and CAIPIRINHA. The reconstructed parametric maps had overall good image quality, accurate quantification, and reasonable noise amplification. Prospective data was obtained and reconstructed and showed consistent quality compared with retrospective data reconstruction. The results show that SUPER-CAIPIRINHA is a feasible and accurate acceleration method for 3D VFA T1 mapping.Acknowledgements
No acknowledgement found.References
1. K FE, J HR, A JG, et al. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magnetic resonance imaging 1987;5(3):201-208.
2. Deoni SCL, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magnetic Resonance in Medicine 2003;49(3):515-526.
3. Cheng H-LM, Wright GA. Rapid high-resolution T1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magnetic Resonance in Medicine 2006;55(3):566-574.
4. Vrenken H, Geurts JJG, Knol DL, et al. Whole-Brain T1 Mapping in Multiple Sclerosis: Global Changes of Normal-appearing Gray and White Matter. Radiology 2006;240:811-820.
5. Arthur C, Fawzi B, Alexandre V, et al. Tissue sodium concentration and sodium T1 mapping of the human brain at 3 T using a Variable Flip Angle method. Magnetic resonance imaging 2019;58:116-124.
6. Menke RA, Scholz J, Miller KL, et al. MRI characteristics of the substantia nigra in Parkinson's disease: A combined quantitative T1 and DTI study. NeuroImage 2009;47(2):435-441.
7. Li Z, Sun J, Hu X, et al. Assessment of liver fibrosis by variable flip angle T1 mapping at 3.0T. Journal of Magnetic Resonance Imaging 2016;43(3):698-703.
8. Ding Y, Rao S-X, Zhu T, Chen C-Z, Li R-C, Zeng M-S. Liver fibrosis staging using T1 mapping on gadoxetic acid-enhanced MRI compared with DW imaging. Clinical Radiology 2015;70(10):1096-1103.
9. Chen B-B, Shih TT-F. DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World Journal of Gastroenterology 2014;20(12):3125-3134.
10. Ippolito D, Inchingolo R, Grazioli L, et al. Recent advances in non-invasive magnetic resonance imaging assessment of hepatocellular carcinoma. World Journal of Gastroenterology 2018;24(23):2413-2426.
11. Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med 2006;55(3):549-556.
12. Hu C, Peters DC. SUPER: A blockwise curve-fitting method for accelerating MR parametric mapping with fast reconstruction. Magnetic Resonance in Medicine 2019;81(6):3515-3529.
Figures