2601
Simultaneous Myelin Water, Magnetic Susceptibility, and Morphometry Analyses Using Magnetization-prepared Multiple Spoiled Gradient Echo1Department of Integrated Health Sciences, Nagoya University Graduate School of Medicine, Nagoya, Japan, 2Department of Radiology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan, 3Department of Neurology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan, 4Department of Rehabilitation Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan, 5Department of Radiology, Nagoya City University Hospital, Nagoya, Japan
Synopsis
This study confirmed the feasibility of the simultaneous acquisition and voxel-based myelin water fraction (MWF), quantitative susceptibility mapping (QSM), and morphometry (VBM) analysis using magnetization-prepared multiple spoiled gradient echo (MP-mSPGR) sequence throughout comparisons with patients with Alzheimer’s disease and healthy control. As a result, the voxel-based MWF could detect demyelination in patients with AD. In contrast, there was not a significant change in susceptibility. This result suggested that the MWF depended on only the myelin content. The VBM analysis delineated the atrophy pattern of AD. The MP-mSPGR sequence is feasible for simultaneous MWF, QSM, and VBM analyses.
Introduction
Myelin water fraction (MWF) can provide a biologically-specific measure of myelin content using multi-component T2* decay model of gradient echo sequence.1 However, recent report has demonstrated that the MWF is accounted for by both myelin content and iron deposition.2 It is needed to simultaneously estimate myelin content and iron deposition such by quantitative susceptibility mapping (QSM). We previously reported a simultaneous voxel-based analysis of QSM and morphometry on single scan using magnetization-prepared multiple spoiled gradient echo sequence (MP-mSPGR).3 This study aimed to confirm the feasibility of a simultaneous voxel-based MWF, magnetic susceptibility and morphometry analysis using MP-mSPGR in elderly healthy control (HC), and patients with Alzheimer’s disease (AD).Material and Methods
1. SubjectOn 3.0 T MRI (Ingenia 3 T; Philips Medical Systems International), MP-mSPGR sequence of 38 patients with AD and 19 HC (mean age: 80 ± 6 and 71 ± 5 years, respectively) were obtained.
2.Data acquisition
The MP-mSPGR sequence serves multiple phase images and strong T1-weighted magnitude images owing to inversion pulse and provides dataset for voxel-based morphometry, QSM, and MWF on a single scan. MP-mSPGR was used with the following imaging parameters: field of view, 192 × 192 × 140 mm; matrix, 192 × 192 × 140; number of TEs, 5; TE1, 6.0 ms; ∆TE, 6.2 ms; TR, 35 ms; flip angle, 15°; inversion time; 1200 ms; shot interval, 2400 ms.
3. Myelin water fraction analysis
To correct the excessive signal loss in the magnitude images due to a macroscopic filed inhomogeneity4, a sinc function and a first-order approximation of the background field gradient estimated by phase images5 was used. The T2* distribution was fitted from the corrected magnitude images to the multiexponential T2* decay, expressed by below equation, using a non-negative least square algorithm with a Tikhonov regularization as a smoothing constraint on a pixel-by-pixel basis4.
$$S_{i}=\sum_{j=1}^MS_{j}e^{-TE_{j}/T2^{*}_{j}}, i=1,2,\cdot\cdot\cdot,N$$
where N is the total number of acquired data points; T*2, j and TEj are the M simulated T2* relaxation times and TEs, respectively; and Sj is the amplitude of the component at each corresponding T2* relaxation time. The simulated T2* values were from 6.0 to 300 ms. The upper T2* bound for myelin water components were defined as the areas of the simulated T2* distributions under 25 ms for myelin water.
4. Quantitative susceptibility mapping
To estimate susceptibility map, the multiple phase images were processed by Laplacian-based phase unwrapping6, sophisticated harmonic artifact reduction of phase data with varying kernels7, and iLSQR algorithm8,9 with zero reference of mean susceptibility in lateral ventricles.10
5. Simultaneous voxel-based analysis
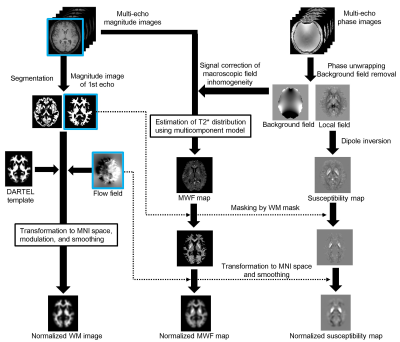
Fig. 1 visually summarizes a procedure of the simultaneous voxel-based analysis.
For VBM, the magnitude image of the first echo in MP-mSPGR was segmented into WM and gray matter. WM images were spatially normalized, smoothed. For voxel-based MWF and magnetic susceptibility analyses, MWF and susceptibility maps were normalized and smoothed using the same parameter used for VBM without any image registration. The groups were compared in terms of the covariates of age, sex, and orientations of the head against the main magnetic field to minimize the influence of the myelin fiber against the main magnetic field on the MWF and QSM. The whole-brain comparisons with family-wise error-corrected P < 0.05 at the cluster-wise level were used to determine regional differences in MWF, susceptibility, and WM volume.
Results
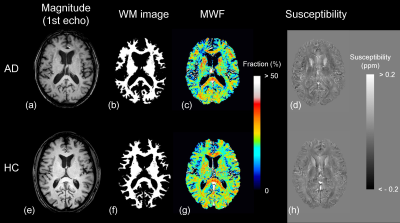
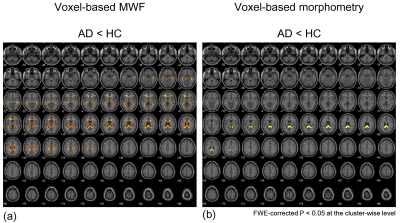
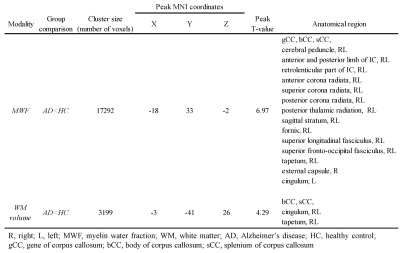
The WM image, MWF, and susceptibility maps were able to reconstruct from a single dataset of MP-mSPGR (Figure 2). In voxel-based MWF analysis, the MWF in patients with AD was significantly decreased that in HC in many WM regions (Figure 3a). In contrast, voxel-based magnetic susceptibility for WM cannot detect any increasing and decreasing susceptibility. Moreover, significant decreases of WM volume in patients with AD was observed, compared with that of HC (Figure 3b). The significant different regions in voxel-based group comparisons are summarized in Table 1.Discussion
In the voxel-based MWF analysis, the significant decreases of MWF in AD indicated demyelination because the vulnerability of oligodendrocytes under Alzheimer’s pathology enables the induction of demyelination since the earlier stage of AD.11 Based on the principle of MWF analysis, the influence of both iron deposition in the WM and the water trapped between the myelin bilayers on the MWF is inevitable2. However, there was no significant increase and decrease in the voxel-based susceptibility analysis of this study. This result suggested that the MWF depended on only the myelin content. The result of VBM analysis delineated the atrophy pattern of AD.3In terms of MWF quantitativeness, the inversion pulse using MP-mSPGR may lead to overestimating MWF because extracellular water with longer T1 relaxation time12 becomes smaller magnetization due to the inversion pulse, compared with myelin water. Further study is necessary to explore this effect. Despite this, the simultaneous acquisition of a dataset for MWF, QSM, and morphometry on a single scan would be advantageous to provide greater spatial accuracy of voxel-based analysis and to estimate the influence of iron deposition against MWF change.
Conclusion
MP-mSPGR sequence is feasible for voxel-based MWF, magnetic susceptibility, and morphometry and highlighting the potential for evaluation of neurodegenerative diseases such as AD.Acknowledgements
This work was supported by KAKENHI, Grant-in-Aid for Scientific Research on Innovative Areas, “Willdynamics” [grant number 16H06403]; the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant number 15K09355] (Y.U) and KAKENHI [grant number 17K15805] (H.K).References
1. Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: A technical review. Magn Reson Med 2015;73(1):70-81.
2. Birkl C, Birkl-Toeglhofer AM, Endmayr V, Hoftberger R, Kasprian G, Krebs C, Haybaeck J, Rauscher A. The influence of brain iron on myelin water imaging. Neuroimage 2019;199:545-552.
3. Kan H, Uchida Y, Arai N, Ueki Y, Aoki T, Kasai H, Kunitomo H, Hirose Y, Matsukawa N, Shibamoto Y. Simultaneous voxel-based magnetic susceptibility and morphometry analysis using magnetization-prepared spoiled turbo multiple gradient echo. NMR Biomed 2020;33(5):e4272.
4. Alonso-Ortiz E, Levesque IR, Paquin R, Pike GB. Field inhomogeneity correction for gradient echo myelin water fraction imaging. Magnetic Resonance in Medicine 2017;78(1):49-57.
5. Kan H, Kasai H, Arai N, Kunitomo H, Hirose Y, Shibamoto Y. Background field removal technique using regularization enabled sophisticated harmonic artifact reduction for phase data with varying kernel sizes. Magn Reson Imaging 2016;34(7):1026-1033.
6. Bagher-Ebadian H, Jiang Q, Ewing JR. A modified Fourier-based phase unwrapping algorithm with an application to MRI venography. J Magn Reson Imaging 2008;27(3):649-652.
7. Wu B, Li W, Guidon A, Liu C. Whole brain susceptibility mapping using compressed sensing. Magnetic Resonance in Medicine 2012;67(1):137-147.
8. Kan H, Arai N, Kasai H, Kunitomo H, Hirose Y, Shibamoto Y. Quantitative susceptibility mapping using principles of echo shifting with a train of observations sequence on 1.5T MRI. Magn Reson Imaging 2017;42:37-42.
9. Li W, Wang N, Yu F, Han H, Cao W, Romero R, Tantiwongkosi B, Duong TQ, Liu C. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage 2015;108:111-122.
10. Liu Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magnetic Resonance in Medicine 2018;79(5):2795-2803.
11. Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer's disease and mild cognitive impairment. Neuroscience 2014;276:206-215.
12. Shin HG, Oh SH, Fukunaga M, Nam Y, Lee D, Jung W, Jo M, Ji S, Choi JY, Lee J. Advances in gradient echo myelin water imaging at 3T and 7T. Neuroimage 2019;188:835-844.
Figures