2588
Disturbed interhemispheric functional and structural connectivity in Type 2 diabetes1Department of Radiology, Zhongda hospital, Southeast University, Nanjing, China, 2Southeast University, Nanjing, China
Synopsis
This study combined the resting-state functional MRI (rs-fMRI) and diffusion tensor imaging (DTI) to explore whether the interhemispheric coordination is altered in type 2 diabetes (T2DM). Results showed that the occipital lobe was disrupted in both functional and anatomic connections and such alteration is strongly associated with endocrine parameters, especially for insulin resistance.
Introduction
Type 2 diabetes (T2DM) is recognized as a serious public health concern and has become a worldwide leading cause for disability and decreased life quality. In recent years, deficits in higher-order cognitive functions have been observed in T2DM patients, but the etiology remains elusive (1). Numerous neuroimaging studies were performed to explore the abnormalities in the diabetic brain, suggesting that the detrimental effects of T2DM on central nervous system are extensive and multifaceted involving both morphology and physiology (2, 3). The two cerebral hemispheres are highly functional interconnected, which is the intrinsic characteristic of the human brain and is highly related to information interaction and communication (4). Although the corpus callosum (CC) have been reported to be affected in T2DM, little is known about the interhemispheric functional synchronization and its associated anatomic connectivity. In the current study, the rs-fMRI and DTI approaches were applied, while voxel-mirrored homotopic connectivity (VMHC) was calculated and the fibers connecting regions with abnormal VMHC were extracted to explore their difference between T2DM patients and age-matched healthy controls (HC). Furthermore, we also examined whether these brain alterations were related to cognitive performance and endocrine parameters.Methods
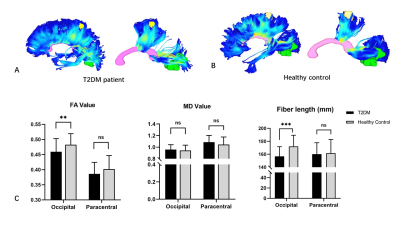
This study included 38 T2DM patients and 42 HCs matched for age, gender and years of education. All subjects underwent extensive neuropsychological assessments for general mental status and specific cognitive domains. MR exams were performed on a 3 T MRI scanner (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. After preprocessing procedures, VMHC value was calculated based on the functional images and compared between the two groups. Regions with significant group difference were set as regions of interest (ROIs) to explore their structural connections. Briefly, fiber bundles connecting the symmetrical ROIs in each hemisphere were extracted from the whole brain fibers derived from the deterministic tractography. The mean FA and MD values and fiber length were extracted and compared between the two groups. Correlations between these metrics and clinical parameters were also investigated, with age, gender, education and score on white matter hyperintensities as covariates.Results
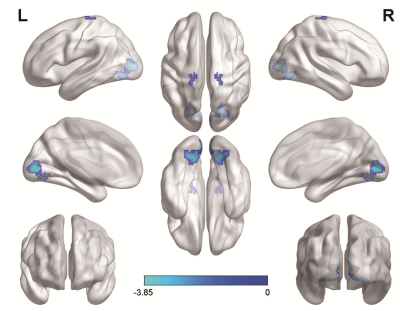
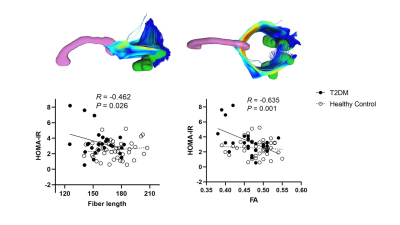
T2DM patients had lower VMHC between bilateral cuneus (occipital lobe) and paracentral lobules. No increased VMHC was found during the comparison. The fibers connecting bilateral cuneus in T2DM group showed significant lower FA and shorter fiber length, suggesting simultaneous impairments in structural connectivity in the occipital lobe. Importantly, insulin resistance was significantly correlated with both the fiber length (r = -0.462, P = 0.026) and the FA value in the fibers connecting bilateral cuneus (r = -0.635, P = 0.001).Discussion & Conclusions
By combining the rs-fMRI and DTI techniques, we investigated the interhemispheric functional as well as anatomical connectivity in T2DM patients. The multi-modality data demonstrated specific disruptions of interhemispheric coordination in the cuneus, both functionally and structurally. Our results not only imply the importance of interhemispheric coordination in the neural substrate in T2DM-related cognitive decline, but also highlighted IR as a promising therapeutic target to reduce the incidence of cognitive impairment.Acknowledgements
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. The study was approved by the local Medical Research Ethics Committee of Zhongda Hospital in accordance with the Helsinki Declaration. Part of the study subjects have been previously reported.References
1. Biessels GJ, Nobili F, Teunissen CE, Simo R, Scheltens P. Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol. 2020;19(8):699-710.
2. Macpherson H, Formica M, Harris E, Daly RM. Brain functional alterations in Type 2 Diabetes - A systematic review of fMRI studies. Front Neuroendocrinol. 2017;47:34-46.
3. Sanjari Moghaddam H, Ghazi Sherbaf F, Aarabi MH. Brain microstructural abnormalities in type 2 diabetes mellitus: A systematic review of diffusion tensor imaging studies. Front Neuroendocrinol. 2019;55:100782.
4. Roland JL, Snyder AZ, Hacker CD, Mitra A, Shimony JS, Limbrick DD, et al. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A. 2017;114(50):13278-83.
Figures