2586
Glioblastoma Recurrence vs. Radiotherapy Injury: Combined Model of DKI and 11C-MET Using PET/MR May Increase Accuracy of Differentiation1Department of nuclear medicine, Chinese PLA General Hospital, Beijing, China, 22. MR Collaboration, Diagnostic Imaging, Siemens Healthcare, Shanghai, China
Synopsis
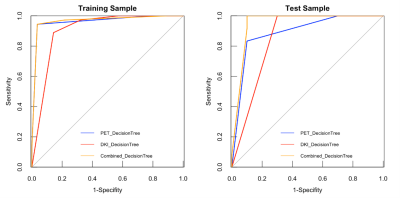
The purpose of this study was to evaluate the diagnostic potential of decision-tree model of diffusion kurtosis imaging (DKI) and 11C-methionine (11C-MET) PET imaging, for the differentiation of radiotherapy injury from glioblastoma recurrence using integrated PET/MR. Eighty-six glioblastoma cases with suspected lesions after radiotherapy were retrospectively enrolled. Compared to models of DKI-alone (AUC=0.85) and PET-alone (AUC=0.89), the combined model demonstrated the best diagnostic accuracy (AUC=0.95). The decision-tree model has the potential to further increase diagnostic accuracy for discrimination between radiotherapy injury and glioblastoma recurrence. 11C-MET PET/MR may thus contribute to the management of glioblastoma patients with suspected lesions after radiotherapy.
Background and purpose
Glioblastoma is the most lethal brain tumor in adults. The 5-year survival rate is less than 5%, and the median survival time after diagnosis is less than 14 months [1]. Even with multimodal therapy, Recurrence or progression is virtually inevitable in glioblastoma during or after treatment [2]. However, the neurological signs and symptoms caused by glioblastoma recurrence and radiotherapy injury are similar, and the differentiation between them imposes challenges on clinical monitoring. Consequently, the correct treatment can be delayed or interrupted [3].Diffusion kurtosis imaging (DKI) is an advanced MRI technique that can characterize the non-Gaussian diffusion of tissue water, which is able to reflect the complexity and heterogeneity of the microstructure in gliomas. Meanwhile, metabolic imaging with 11C-methionine (MET) positron emission tomography (PET) has also been used to indicate the proliferation and metabolism of glioblastoma, adding value to anatomical imaging for diagnosis of early glioblastoma recurrence and thus being beneficial for prompt treatment [4-6]. The purpose of this study was to evaluate the diagnostic potential of decision-tree model of diffusion kurtosis imaging (DKI) and 11C-methionine (11C-MET) positron emission tomography (PET), for the differentiation of radiotherapy injury from glioblastoma recurrence using integrated PET/MR.
Methods
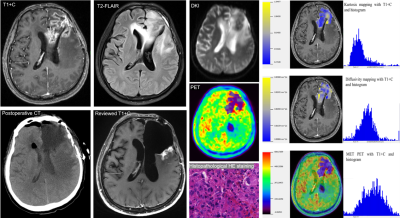
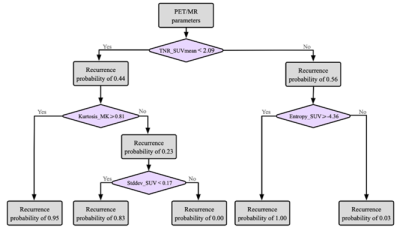
Eighty-six glioblastoma cases with suspected lesions after radiotherapy were retrospectively enrolled. All the patients were scanned on a whole-body PET/MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany) with a 12-channel head coil. The scanning protocol included: (1) 11C-methionine PET examination; (2) 3D T1-weighted magnetization-prepared rapid gradient-echo and contrast enhanced T1-weighted imaging; (3) a transversal T2-weighted fluid-attenuated inversion recovery; (4) a transversal DKI protocol based on a single-shot echo-planar imaging.Based on histopathology or follow-up, 48 patients were diagnosed with local glioblastoma recurrence and 38 patients were radiotherapy injury. All the patients underwent PET/MR examinations. Multiple parameters were derived based on the ratio of tumor to normal control (TNR), including mean and maximum standardized uptake values (SUVmax, SUVmean), mean value of kurtosis and diffusivity (MK, MD) from DKI and histogram parameters. The diagnostic models were established by decision trees. Receiver-operating-characteristic analysis was used for evaluating the diagnostic accuracy of each independent parameter and all the diagnostic models.
Results
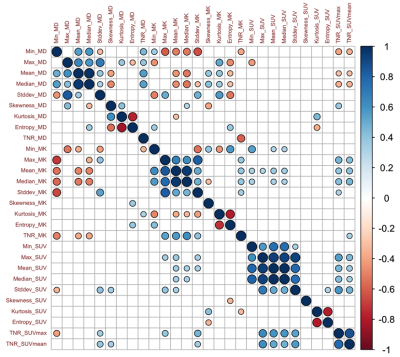
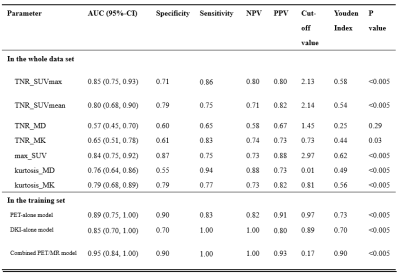
The study finally included 86 patients. In detail, there were 71 patients with maximum safe resection followed by RT; 12 patients with the biopsy followed by hypofractionated RT. Forty-eight patients were diagnosed with glioblastoma recurrence and thirty-eight patients were diagnosed with radiotherapy injury after PET/MR examination. All the cases of glioblastoma recurrence were confirmed by histopathological result; all the cases of radiotherapy injury were diagnosed with either histopathological results or the clinical follow-up.The inter-cluster correlations of DKI-, PET- and texture parameters were relatively weak, while the intra-cluster correlations were strong. Glioblastoma recurrence had significantly higher TNR_SUVmax (3.53±1.49 vs 1.86±0.73, P<0.005), TNR_SUVmean (3.31±1.66 vs 1.86±0.77, P <0.005) and TNR_MK (0.90±0.26 vs 0.83±0.52, P <0.005) than radiotherapy injury. Compared to models of DKI-alone (AUC=0.85) and PET-alone (AUC=0.89), the combined model demonstrated the best diagnostic accuracy (AUC=0.95).
Discussion
In the present study, we investigated the relationships among parameters with 11C-MET PET, DKI and texture feature. The results showed the parameters derived from different approaches provided complementary information about metabolism, cellularity and heterogeneity of the lesion. Our study established differential diagnostic models based on the decision tree with multiple-parameter PET/MR. Compared to models of DKI-alone (AUC=0.85) and PET-alone (AUC=0.89), the diagnostic models with combined parameters improved the differential diagnostic efficacy (AUC=0.95). The integrated model based on the multi-parametric features demonstrated the potential contribution to the management of glioblastoma patients with inconclusive lesions after radiotherapy in postoperative follow-up, as well as improving the prognosis.Regular monitoring of glioblastoma after treatment is usually based on RANO by contrast-enhanced magnetic resonance imaging [7]. Our research has shown the accuracy of 0.791 with CE-MRI. The typical characteristics of glioblastoma recurrence usually appear as the progressive or new lesions on contrast-enhanced T1-weighted imaging or on T2-weighted imaging [8]. The radiotherapy injury manifests as the irregular enhanced lesion around the therapeutic field, with edema in the surrounding gray or white matter [9]. The accurate differentiation between tumor recurrence and radiotherapy injury is crucial for clinical decision-making. For the former patients, biopsy and chemoradiotherapy should be carried out as soon as possible, and anti-inflammatory treatment can be carried out for the latter patients. Unfortunately, using the MR characteristics to distinguish between radiotherapy injury and glioblastoma recurrence is often difficult. Recently, the integrated PET/MR has been shown to provide consistent imaging fusion and advantages of multiparametric analysis from different modalities [10, 11]. This indicates that the quantitative analysis with integrated 11C-MET PET/MR could play a promising role in the timely and conclusive diagnosis of glioblastoma recurrence and radiotherapy injury.
Our study had limitations. First, the size of the training set was relatively small. Second, the samples of radiotherapy injury and glioblastoma recurrence were unbalanced, and the statistical results could therefore be potentially biased.
Conclusions
DKI, 11C-MET PET and histogram parameters provide complementary information about tissue. The decision-tree model combined of theses parameters has the potential to further increase diagnostic accuracy for discrimination between radiotherapy injury and glioblastoma recurrence. 11C-MET PET/MR may thus contribute to the management of glioblastoma patients with suspected lesions after radiotherapy.Acknowledgements
References
[1] Stupp R, Tonn JC, Brada M, Pentheroudakis G, Group EGW. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21 Suppl 5(suppl_5):v190-193.
[2] Glavatskyi O, Zemskova O. RECURRENT GLIOBLASTOMA MANAGEMENT USING STEREOTACTIC RADIOSURGERY AND SYSTEMIC TREATMENT. Technology transfer: innovative solutions in medicine. 2018:15-17.
[3] Lohmann P, Kocher M, Ceccon G, et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. NeuroImage: Clinical. 2018; 20:537-542.
[4] Lucas JT, Serrano N, Kim H, et al. 11 C-Methionine positron emission tomography delineates non-contrast enhancing tumor regions at high risk for recurrence in pediatric high-grade glioma. Journal of neuro-oncology. 2017; 132(1):163-170.
[5] Beppu T, Terasaki K, Sasaki T, et al. MRI and 11C-methyl-L-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clinical nuclear medicine. 2016; 41(11):852-857.
[6] Jung T-Y, Min J-J, Bom H-S, et al. Prognostic value of post-treatment metabolic tumor volume from 11 C-methionine PET/CT in recurrent malignant glioma. Neurosurgical review. 2017; 40(2):223-229.
[7] Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963-1972.
[8] Deuschl C, Kirchner J, Poeppel TD, et al. 11 C–MET PET/MRI for detection of recurrent glioma. European journal of nuclear medicine and molecular imaging. 2018; 45(4):593-601
[9] Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between Treatment-Induced Necrosis and Recurrent Tumors in Patients with Metastatic Brain Tumors: Comparison among (11)C-Methionine-PET, FDG-PET, MR Permeability Imaging, and MRI-ADC-Preliminary Results. AJNR Am J Neuroradiol. 2017; 38(8):1520-1527.
[10] Yoon RG, Kim HS, Koh MJ, et al. Differentiation of recurrent glioblastoma from delayed radiation necrosis by using voxel-based multiparametric analysis of MR imaging data. Radiology. 2017; 285(1):206-213.
[11] Falk Delgado A, Nilsson M, van Westen D, Falk Delgado A. Glioma Grade Discrimination with MR Diffusion Kurtosis Imaging: A Meta-Analysis of Diagnostic Accuracy. Radiology. 2018; 287(1):119-127.
Figures