2578
Alterations in brain connectivity as duration of disease increases in Parkinson's disease.1Neurology, AIIMS, Delhi, India, 2Nuclear Magnetic Resonance, AIIMS, Delhi, India, 3Neurology, Medanta, The Medicity, Gurgaon, India
Synopsis
Parkinson’s disease (PD) is charcterized by progressive degenration of dopaminergic neurons in substantia nigra. This leads to motor symptoms like bradykinesia with rigidity and tremor. PD patients also experience difficulty in traversing through narrow spaces. Indicating towards altered motor execution and planning and decision making. This study was aimed thus to explore the progressively deteriorating brain connectivity in PD. Functional connectivity was observed to be altered with duration of disease.
Introduction:
Parkinson’s disease (PD) is a movement disorder which is progressive in nature. PD patients primarily experience bradykinesia with rigidity and tremor1. Amongst other movement related symptoms there is difficulty in navigating themselves through narrow or congested spaces and this involves an effort from the motor planning and execution areas of the cortex2,3. It is known that treatment with dopamine may have a role in restoring the cortical activity4. This study explored the functional cortical connectivity (cue-dependent movement execution task) with respect to disease progression and clinical presentation of the symptoms.Method:
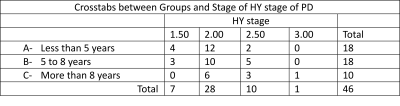
The study was approved by the institute review board. Patients with PD were recruited from the movement disorders clinics of department of Neurology of our institute and were recruited after obtaining their informed written consent in compliance with Declaration of Helsinki. Subjects with idiopathic Parkinson‘s disease as per UK Parkinson‘s disease Brain Bank Criteria of both gender and age above 45 years were chosen to be a part of the study. Patients with PD (n = 46) with Hoehn and Yahr (HY) ≤ 3, HAM A ≤ 14 (but not on any anxiolytics), HAM-D ≤ 7 and right sided onset of the first symptom (tremor, bradykinesia or akinesia, rigidity) were recruited. Participants with contraindications to the fMRI, history of any neuro-psychiatric co-morbidities were excluded. Clinical assessment like UPDRS (Unified Parkinson’s Disease Rating Scale) as well as fMRI sessions were conducted in drug ON phase. Functional MRI data was acquired on a 3 Tesla MRI scanner (Achieva 3.0T TX, M/s. Philips Medical Systems, Netherlands) with a 32 channel head coil. The subjects were scanned in supine position with the head supported and immobilized using vendor provided foam pads within the head coil. The task was generated using the E-prime software (version 1.1, Psychology Software Tools, Inc. USA) and presented during MRI session using a MR compatible LCD monitor (E-sys, M/s Philips). A single shot echo planar imaging (EPI) sequence with number of slices=29, Slice thickness = 5 mm, Slice gap: 0 mm, Slice orientation: transverse, Fold-over direction: RL, multi-sliced-interleaved, Reconstruction matrix: 128, Coil elements: 32, Scan mode technique: fast Fourier echo (FFE), Flip angle = 90°, Field of view (FOV) = 240 mm (RL); 232 mm (AP); with TR/TE =2000/30 ms, number of dynamics 192 was acquired. Task was a block type design with 6 blocks of active alternating with periods of rest. A set of randomly appearing images of a triangles (pointing either right or left signifying a direction) were presented during the active blocks of the task. During a single active block a total of 8 images (each lasting for 4 secs) were presented. Out of these 8 images, 4 were of that of right pointing triangle and 4 that of left pointing triangle (which was randomly presented). Depending upon the direction of the presenting image (pointing towards right or left), subjects were instructed to respond by pressing MR compatible response pad (Lumina 4-key button response). Each block active or rest lasted for 32 seconds each. The fMRI data were analyzed using Conn toolbox5 and demographic data with SPSS 22 (IBM Corp)6. The functional data were realigned, co-registered to the anatomical image, normalized to the template, smoothed (FWHM=8 Hz) and results explores at 2nd level analyses.Result:
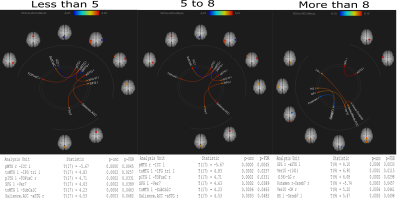
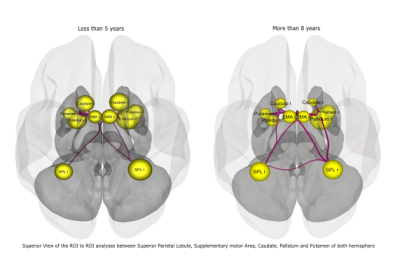
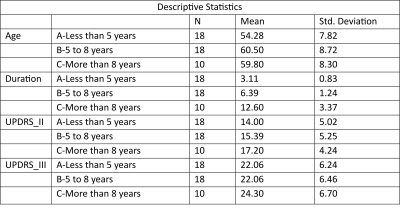
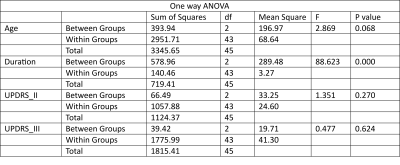
The study was conducted on a total of 46 subjects and then they were categorized into 3 groups depending on the duration of disease: A= less than 5 years of disease; B= 5 to 8 years of disease; C= above 8 years. Seed based connectivity analyses revealed significant differences in group C (Figure 1). ROI to ROI analyses also showed significant difference in group C (Figure 2). No significant differences were observed in age (p=0.068), UPDRS II (p=0.270), UPDRS III (p=0.624) across the groups as revealed by one way anova (Tables 1,2,3).Discussion:
Disease onset was from right side of the body and the results corroborated this by disrupted connectivity in the Left hemispheres7. The task was an externally cued movement execution and thus there were significant activations in the supplementary motor area (SMA) along with parietal association areas. SMA is known to regulate externally generated movement8. With disease progression, a compensatory recruitment of cortical and subcortical nodes imminent to PD9–11, instead of restoration of networks was evident. Involvement of bilateral parietal cortices with the duration of disease suggest plasticity implicating inter-hemispheric connectivity.Conclusion:
The results shed light on the altered functional connectivity in PD as the disease progresses.These differences in connectivity measures are important to monitor disease progress and plan specific treatments and rehabilitation strategies.Acknowledgements
No acknowledgement found.References
1. Fahn, S. Classification of movement disorders. Movement Disorders (2011) doi:10.1002/mds.23759.
2. Fling, B. W., Curtze, C. & Horak, F. B. Gait asymmetry in people with Parkinson’s disease is linked to reduced integrity of callosal sensorimotor regions. Front. Neurol. (2018) doi:10.3389/fneur.2018.00215.
3. Shibley, R., Griffin, H. J., Quinn, N. P. & Jahanshahi, M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov. Disord. (2008) doi:10.1002/mds.21667.
4. Mallol, R. et al. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. (2007) doi:10.1016/j.brainres.2007.02.046.
5. Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. (2012) doi:10.1089/brain.2012.0073.
6. IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, N. I. C. IBM SPSS V22. (2013).
7. Heinrichs-Graham, E., Santamaria, P. M., Gendelman, H. E. & Wilson, T. W. The cortical signature of symptom laterality in Parkinson’s disease. NeuroImage Clin. (2017) doi:10.1016/j.nicl.2017.02.010.
8. Herz, D. M., Eickhoff, S. B., Løkkegaard, A. & Siebner, H. R. Functional neuroimaging of motor control in parkinson’s disease: A meta-analysis. Hum. Brain Mapp. (2014) doi:10.1002/hbm.22397.
9. Whone, A. L., Moore, R. Y., Piccini, P. P. & Brooks, D. J. Plasticity of the nigropallidal pathway in Parkinson’s disease. Ann. Neurol. (2003) doi:10.1002/ana.10427.
10. Kojovic, M. et al. Functional reorganization of sensorimotor cortex in early Parkinson disease. Neurology (2012) doi:10.1212/WNL.0b013e318253d5dd.
11. Grafton, S., Turner, R. & Desmurget, M. Normalizing motor-related brain activity Subthalamic nucleus stimulation in Parkinson disease. Neurology (2006).
Figures