2576
Determining Spinal Cord pH using Chemical Exchange Saturation Transfer (CEST) MRI1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Siemens Healthineers, Erlangen, Germany, 4Clinical Neurological Sciences, University Hospital, London Health Sciences Centre, London, ON, Canada
Synopsis
Degenerative cervical myelopathy (DCM) is one of the most common forms of spinal cord dysfunction. Predicting functional recovery after surgery remains elusive. Pathophysiological mechanisms, like ischemia and hypoxia in the spinal cord, could impact recovery after surgery. Chemical Exchange Saturation Transfer (CEST) produces image contrast based on the rate of exchange of amine and amide protons. This exchange rate is dependent on tissue pH, creating a pH-weighted contrast. CEST imaging in the spinal cord incorporating respiratory correction could be used to examine tissue pathology caused by hypoxia in DCM and other spinal cord injuries.
Introduction
Degenerative cervical myelopathy (DCM) is a unique model of spinal cord injury that can result in spinal cord compression and neurological dysfunction.1 The incidence and prevalence in North America are estimated to be 41 and 605 per million, respectively,2 making it one of the most common forms of spinal cord dysfunction. It has been hypothesized that pathophysiological mechanisms, like ischemia and hypoxia in the spinal cord, could impact recovery after surgery but direct in-vivo evidence of such changes has been limited in humans. Chemical Exchange Saturation Transfer (CEST) is an MRI contrast method that produces image contrast based on the rate of exchange between protons in amine and amide groups within tissue and bulk water protons as an example. Since exchange rate is pH dependent, CEST images can be acquired with pH-weighting using a ratiometric method called amine/amide concentration-independent detection (AACID).3 However, the spinal cord is in close proximity to the lungs. Consequently, CEST image acquisition is complicated by motion artefacts and magnetic susceptibility changes during data acquisition that cause signal fluctuations. Spinal cord CEST could have widespread applications in understanding disease processes; however, a method to overcome these artefacts must be developed. The objective of this study is to characterize the reproducibility of AACID CEST MRI on a 3.0 T Siemens Prisma system incorporating a previously published respiratory correction method.4Methods
On a 3.0 T Siemens Prisma Fit MRI scanner, a CEST sequence is used which incorporates a single slice 2D gradient echo (GRE) readout. In the spine, CEST saturation was performed with 21 Gaussian shaped pulses (B1 = 2.0 µT, total saturation time of 1.9 sec), applied at 91 offsets from -6.5 ppm to 6.5 ppm. Other relevant parameters include: TR/TE = 30.0/4.4 msec, voxel size = 1.5 mm x 1.5 mm, slice thickness = 5 mm, 1 average and fat suppression applied. 30 non-saturated scans were interleaved throughout the acquisition to track the variation of intensity resulting from the global effect of respiration. The non-saturated scans were spline-interpolated and used to normalize the signal intensity of the CEST spectrum. To correct for signal fluctuations due to respiration, the respiratory cycle was monitored throughout the CEST acquisition using the respiratory bellows to determine respiration volume per unit time (RVT), defined by dividing the range of the cycle magnitude by the mean respiration time between peaks for every CEST image collected.4 A respiratory correction was applied by scaling the RVT by the range of the signal drift in the non-saturated images to regress signal variation due to breathing out of the data.4 To correct for B0 field inhomogeneities, the CEST spectrum was shifted on a pixel-by-pixel basis using a water saturation shift referencing (WASSR) scan.5 For WASSR, 5 Gaussian shaped pulses, each at 0.5 µT, were applied at 21 offsets from -2.0 ppm to 2.0 ppm with the following parameters: TR/TE = 10.0/4.4 msec, voxel size = 1.5 mm x 1.5 mm and 1 average. Healthy subjects were recruited to evaluate the pulse sequence and the respiratory correction in the spinal cord. Initial optimization of the AACID CEST imaging method was completed in the brain of a healthy subject. Further optimization was performed in the spinal cord using the retrospective respiratory correction algorithm implemented in MATLAB.Results
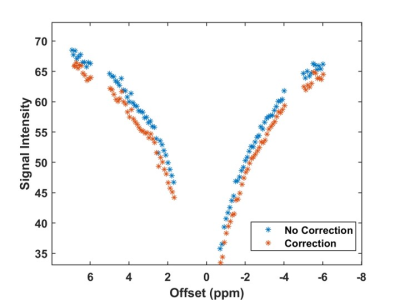
Figure 1 demonstrates a spinal cord CEST image and CEST signal intensity variation within the cord during the acquisition with interleaved non-saturated scans. The non-saturated scans had a coefficient of variation of 1.3%. Figure 2 demonstrates the CEST spectrum in the spinal cord with no respiratory correction applied and the respiratory correction applied, demonstrating a slight improvement in the visibility of the amide CEST effect at 3.5 ppm.Discussion
The CEST sequence produced high quality images in the spinal cord. The interleaving of the non-saturated scans demonstrated global signal variation due to breathing. The CEST spectra collected in the spinal cord are reminiscent of those acquired in the brain. The respiratory correction method appears to increase the visibility of the amide CEST effect likely increasing the accuracy of in-vivo pH measurement.Conclusion
Although the application of CEST in the spinal cord has been demonstrated previously, here we utilize a prototype CEST sequence on a Siemens Prisma Fit combined with respiratory correction. Further optimization of the spinal CEST sequence is required to further improve the fidelity of the measurements. In the future, this correction method will be tested on healthy subjects to determine the reproducibility of pH-weighted measurements in the spinal cord using amine/amide concentration independent detection (AACID). AACID CEST will then be performed on DCM patients to examine the heterogeneity of pH in the spinal cord near the site of compression.Acknowledgements
The authors thank all participants for their contribution to this project. We also thank Scott Charlton and Oksana Opalevych (CFMM, Robarts Research Institute, The University of Western Ontario) for facilitating MRI acquisitions.References
[1] Toledano M, Bartleson JD. (2013). Cervical spondylotic myelopathy. Neurol Clin 2013; 31: 287-305.
[2] Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015; 40: E675-693.
[3] McVicar N, Li AX, Gonçalves DF, Bellyou M, Meakin SO, Prado MAM, Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow & Metab 2014; 34: 690-698.
[4] By S, Barry RL, Smith AK, Lyttle BD, Box BA, Bagnato FR, Pawate S, Smith SA. Amide Proton Transfer CEST of the Cervical Spinal Cord in Multiple Sclerosis Patients at 3T. Magn Reson Med 2018; 79: 806-814.
[5] Kim M, Gillen J, Landman BA, Zhou J, van Zijl PCM. Water Saturation Shift Referencing (WASSR) for Chemical Exchange Saturation Transfer (CEST) Experiments. Magn Reson Med 2009; 61: 1441-1450.
Figures
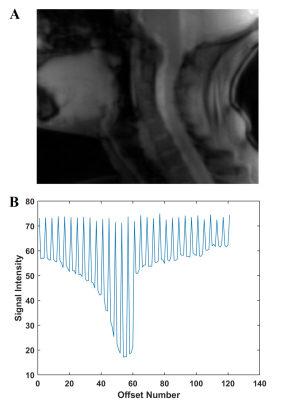
Figure 1:
A: Raw sagittal spinal cord CEST image.
B: CEST spectrum of spinal cord with the interleaved non-saturated scans demonstrating the global respiratory effect.