2571
Increased functional connectivity but intact memory performance in tauopathy mouse at pre-tangle stage1Queensland Brain Institute, University of Queensland, St Lucia, Australia, 2Clem Jones Centre for Ageing Dementia Research, University of Queensland, St Lucia, Australia, 3Centre for Advanced Imaging, University of Queensland, St Lucia, Australia
Synopsis
Although pre-tangle stage of tauopathy is regarded as an important pathogenic factor in neurodegenerative disease, their relationship with brain functional connectivity (FC) is still ambiguous. We investigated a tau mouse (rTg4510) model at 2.5 month-old age by resting-state functional MRI, spatial memory task and histopathology. We found increased FC between hippocampus and medial temporal area in rTg4510. Moreover, increased FC is negatively related to learning performance even though the memory is intact. On pathology, there is little phosphorylated tau over temporal areas. Our results suggest FC alternation may be an early sign of neural dysfunction at pre-tangle stage.
Introduction
Tauopathies are pathological hallmarks of various neurodegenerative diseases, including Alzheimer's disease (AD)1. Studies have shown that the type of cognitive decline is related to specific brain regions affected by tauopathy2. Recently, studies revealed that hyper-phosphorylated tau oligomer is toxic to neurons and would affect neuronal excitability in transgenic mice, suggesting that pathognomonic forms are established before tangles3, 4. Although human studies have shown that alteration of functional connectivity (FC) and tauopathy has topographical correlation, how FC is affected at pre-tangle stage and what are their relationships with cognition are still unknown. Here, we conducted a study in a young transgenic mouse model of tauopathy before tangle is formed using functional MRI, spatial memory task and histopathology.Method
All the experiments were approved by the animal ethics committee of the University of Queensland. rTg(TauP301L)4510 (n=16, male) and their littermate controls (n=14, male) were used. MRI was done at 10-12 week-age old. MRI was conducted on a 9.4T preclinical scanner (Bruker BioSpin MRI GmbH, Germany) under low dose of isoflurane (0.1-0.3% via nosecone) and medetomidine (0.1mg/Kg/h, ip). To ensure stable neurovascular coupling, visual cortex response to visual stimulation was confirmed before acquiring resting-state functional imaging for 10 minutes. A multiband EPI of TR/TE=300/15ms was used5. An active place avoiding (APA) task was conducted 1 week after MRI to assess the spatial memory6. Each APA is comprised of 5 trials (10 mins per trial). Mice have to learn spatial cues as a reference to avoid entering a shock zone in a rotating arena. The error number of entrance into shock zone and maximum avoidance time (MAT) between 2 shocked episodes per trial were used to evaluate memory performance. Immunohistochemistry was done using AT8 and pSer422 for identifying phosphorylated tau. For fMRI analysis, an optimal preprocessing pipeline was used for nuisance removal7. EPI was coregistered to the AMBMC template. Seed-based FC analysis was done using anatomical ROI labelled on the AMBMC atlas. The correlation matrix was transformed to Fisher-Z value for two-sample t-test at the group level. Significant level was set p<0.0005 uncorrected. Significant FC was correlated with error number change or MAT change using spearman correlation separately in each group to avoid disease effect. Behaviour was compared by t-test. All statistical analysis was completed on SPSS ver22.0.Results
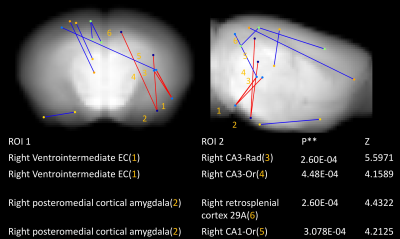
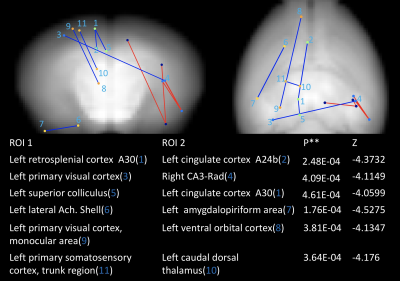
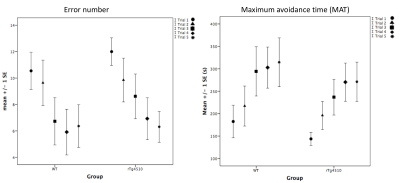
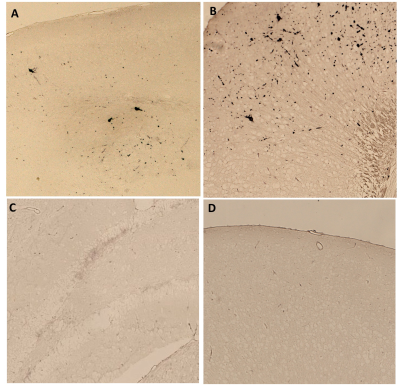
Compared to littermates, rTg4510 mice showed significantly increased FC between the right hippocampus (CA3) and right medial temporal area (entorhinal cortex and posteromedial cortical amygdala (PMCo)); between the right hippocampus (CA1) and right anterior cingulate cortex. Decreased FC between the left frontal lobe and other areas was found (Figure 1, 2). For behaviour, there is no significant difference between groups (Figure 3). Interestingly, we found a negative correlation between FC and MAT or error number change in rTg4510 mice but not littermates (Figure 4). Histochemical staining showed scarce phosphorylated tau stained over the hippocampal area and entorhinal cortex, but more prominent over frontal and cingulate cortex (Figure 5).Discussion
Our pathological result is consistent with the fact that olfactory bulb and frontal lobe are the earliest brain region having pathological deposition. This is in line with decreased FC with the frontal area. We found increased FC between ipsilateral entorhinal cortex, CA3, CA1 and PMCo. PMCo has dense connection with accessory olfactory bulb and ventral hippocampus (e.g., CA1)8. Entorhinal cortex also has reciprocal connection with the hippocampus. The increased cortical FC may be an early phenomenon preceding pathological tau deposition. This is in contrast to disrupted FC in regions with more prominent pathology. Particularly, we found that the hyper-FC is negatively correlated with the learning performance during the spatial memory task while there was no behavioural impairment at this stage. Such negative correlation is in accordant with the human study indicating failure of compensation predicts future rapid deterioration9. Longitudinal follow-up is warranted to clarify this hypothesis.Acknowledgements
We thank Professor Jürgen Götz, chair of Clem Jones Centre for Ageing Dementia Research, Queensland Brain Institute for providing transgenic mice in this study as a gift.References
1. Scheltens, P., et al., Alzheimer's disease. Lancet, 2016. 388(10043): p. 505-17.
2. Chong, F.P., et al., Tau Proteins and Tauopathies in Alzheimer's Disease. Cell Mol Neurobiol, 2018. 38(5): p. 965-980.
3. Arendt, T., J.T. Stieler, and M. Holzer, Tau and tauopathies. Brain Res Bull, 2016. 126(Pt 3): p. 238-292.
4. Lasagna-Reeves, C.A., et al., Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener, 2011. 6: p. 39.
5. Lee, H.L., et al., Ultrafast fMRI of the rodent brain using simultaneous multi-slice EPI. Neuroimage, 2019. 195: p. 48-58.
6. Willis, E.F., P.F. Bartlett, and J. Vukovic, Protocol for Short- and Longer-term Spatial Learning and Memory in Mice. Front Behav Neurosci, 2017. 11: p. 197.
7. Chuang, K.H., et al., Evaluation of nuisance removal for functional MRI of rodent brain. Neuroimage, 2019. 188: p. 694-709.
8. Gutierrez-Castellanos, N., et al., The vomeronasal cortex - afferent and efferent projections of the posteromedial cortical nucleus of the amygdala in mice. Eur J Neurosci, 2014. 39(1): p. 141-58.
9. Dickerson, B.C., et al., Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol, 2004. 56(1): p. 27-35.
Figures