2570
Neurite Orientation Dispersion and Density Imaging in a Pre-clinical Model of Repetitive Mild Traumatic Brain Injury1Patrick McCunn, SickKids Research Institute, Toronto, ON, Canada, 2Robarts Research Institute, London, ON, Canada
Synopsis
Identification of immediate microstructural changes following repetitive mild traumatic brain injury (mTBI) could shed light on the pathophysiology of second impact syndrome. The purpose of this study was to apply Neurite Orientation Dispersion and Density Imaging (NODDI) to a preclinical model of repetitive mTBI. In the corpus callosum, neurite density index (NDI) showed a significant increase within two hours following both the first and second injury, while orientation dispersion index (ODI) showed an increase after the first injury only. These results suggest an early microstructural response to repetitive mTBI which may follow a dose-dependent like response.
Introduction
Repetitive mild traumatic brain injury (mTBI) has become a major health concern over the past few decades with a substantial increase in the awareness of the detrimental health effects associated with this injury [1]. Unfortunately very little is understood about the underlying pathophysiological features of repetitive mTBI [2–4]. Previous work in our lab showed that NODDI was successfully able to detect changes in neurite density index (NDI) and orientation dispersion index (ODI) within the first two hours post injury in the corpus callosum and hippocampus. Here we applied NODDI to a pre-clinical model of repetitive mTBI. We hypothesize that NODDI would be sensitive to changes in the microstructural environment of rodents within the first hour of both a primary and a secondary mild traumatic brain injury.Methods
Twelve adult male Wistar rats (injury: n = 8, age at baseline scan: 112 ± 13 days, weight at baseline scan: 278 g ± 38 g, control: n = 4, age at baseline scan: 123 ± 24 days, weight at baseline scan: 313 ± 51 g) were scanned on a Bruker 9.4 Tesla small animal MRI using a multi-shell acquisition. Eight animals experienced two closed head controlled cortical impacts followed immediately by NODDI from 1-2 hours post injury. Four sham control rats were scanned without injury. NODDI acquisition parameters included: Shell one: 30 directions, b-value = 1000 s/mm2, G = 172.85 mT/m, Δ = 14 ms, the duration of a single gradient pulse (δ) = 4.5 ms; Shell two: 60 directions, b-value = 2000 s/mm2, G = 345.70 mT/m, Δ= 14 ms, δ = 4.5 ms. Ten b = 0 s/mm2 images were interspersed evenly throughout the acquisition. Four averages were used to ensure adequate signal to noise ratio (SNR) in the higher b-value shell. The NODDI diffusion sequence was incorporated into a multi-shot, spin echo, echo-planar-imaging (EPI) acquisition pulse sequence (4 shots, 32 slices, slice thickness = 500 mm, FOV 40 x 40 mm, matrix size 160 x 160, in-plane resolution = 250 × 250 mm, TE = 26.71 ms, TR = 2.5 s).Images were processed using the fMRI Software Library (FSL, v. 6.0.1, Oxford, UK and the NODDI Matlab toolbox (available from the University College London (UCL) Microstructure Imaging Group). Region of interest (ROI) analysis focused on the corpus callosum and hippocampus. A mixed ANOVA was used to determine statistically significant interactions in NDI, ODI, fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity. Follow up repeated-measures ANOVA’s were used to determine individual changes over time.
Results
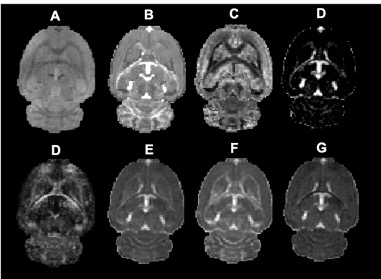
Data were successfully acquired from all 12 subjects. Figure 2 depicts single subject raw data, NODDI scalar maps, and DTI scalar maps. The following timepoints were used in this study: Timepoint 1 (or baseline scan) obtained 7 days prior to injury, Timepoint 2 began at 62 ± 14 minutes post-injury 1, Timepoint 3 began at 98 ± 3 hours post-injury, and Timepoint 4 began at 66 ± 11 minutes post-injury 2.Corpus Callosum
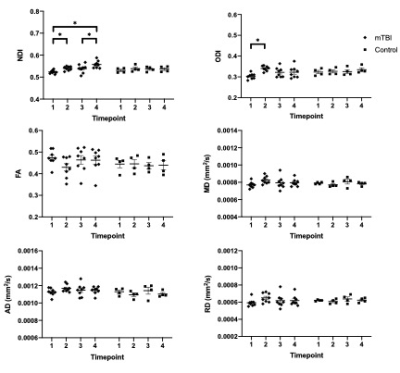
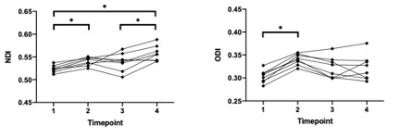
In the corpus callosum, there was a statistically significant (p < .05) group by time interaction effect for NDI [F(3,30) = 4.161, p = .033, Greenhouse-Geisser corrected] and ODI [F(3,30) = 3.851, p = .047, Greenhouse-Geisser corrected]. There was no statistically significant interaction effect (p > .05) of group by time for FA, MD, AD and RD. From the subsequent univariate repeated measures ANOVA, it can be seen that for both NDI and ODI there was a statistically significant change over time for the injured group, while no statistically significant change over time occurred within the control group for these metrics. Similar to our previous study, it can be seen that for every individual subject both ODI and NDI increased after the initial mTBI. Using a two-tailed paired t-test it was shown that for NDI there was no statistically significant difference in the response to injury 1 and injury 2 in the corpus callosum (p = .68). For ODI there was a statistically significant difference in the response to injury 1 and injury 2 (p =.00)
Hippocampus
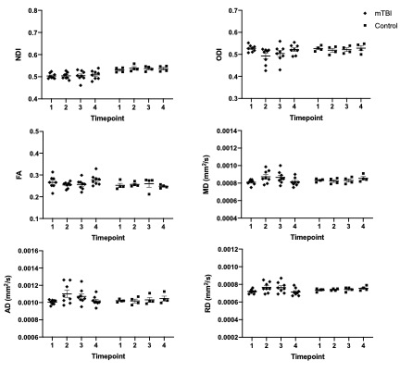
In the hippocampus, there was no statistically significant interaction effect (p > .05) of group by time for NDI, ODI, FA, MD, AD and RD.
Conclusion
NODDI was successfully able to detect changes in NDI and ODI in the brain of rodents immediately following a first and a second closed skull controlled cortical impact. The measured changes largely did not differ between the first and second impact when comparing groups but varied dramatically on an individual basis. This leads us to conclude the immediate response to repetitive mTBI is extremely heterogeneous and could provide clues with regard to the varied recovery rates evident in the clinical patient populationAcknowledgements
No acknowledgement found.References
1. Dixon KJ. Pathophysiology of Traumatic Brain Injury. Physical Medicine and Rehabilitation Clinics of North America. 2017. doi:10.1016/j.pmr.2016.12.001
2. Prigatano GP, Gale SD. The current status of postconcussion syndrome. Curr Opin Psychiatry. 2011; doi:10.1097/YCO.0b013e328344698b
3. Ojo JO, Mouzon BC, Crawford F. Repetitive head trauma, chronic traumatic encephalopathy and tau: Challenges in translating from mice to men. Experimental Neurology. 2016. doi:10.1016/j.expneurol.2015.06.003
4. Bailes JE, Dashnaw ML, Petraglia AL, Turner RC. Cumulative effects of repetitive mild traumatic brain injury. Concussion. 2012. doi:10.1159/000358765
Figures