2569
A fiber clustering-based atlas of the chimpanzee deep brain structural connectivity using diffusion MRI
Maëlig Chauvel1, Ivy Uszynski1, William Hopkins2, Jean-François Mangin1, and Cyril Poupon1
1Université Paris-Saclay, CEA, CNRS, BAOBAB, Neurospin, Gif-sur-Yvette, France, 2Keeling Center for Comparative Medicine and Research, The University of Texas MD Anderson Cancer Center, Bastrop, TX, United States
1Université Paris-Saclay, CEA, CNRS, BAOBAB, Neurospin, Gif-sur-Yvette, France, 2Keeling Center for Comparative Medicine and Research, The University of Texas MD Anderson Cancer Center, Bastrop, TX, United States
Synopsis
A way to better appreciate the ancient or evolved Homo sapiens brain characteristics relies on comparative investigations with homologous species. Chimpanzees remain our closest living hominid relatives, making it a pertinent model for brain studies. Few investigations address the structural connectivity of the chimpanzee brain, whereas its proximity with humans could be key to understanding the human brain. Thanks to the unique imaging data collection provided by the National Yerkes Primate Research Center and the access to elaborated diffusion MRI tools to investigate brain connectivity, we propose a novel long bundle atlas of the white matter of the chimpanzee brain.
Introduction
Mapping the chimpanzee brain connectome is a key to the comprehension of the singularity of human brain evolution as it is our closest phylogenetic hominid cousin1. Contrary to the macaque species, few studies have been performed in vivo on chimpanzees due to this proximity with humans which pushes Ethical Committees to prevent their use for research. Most existing imaging studies on chimps focus on anatomy2 and asymmetry of brain structures3. While human atlases of the structural connectivity exist for humans4-5, only one study6 has addressed this topic in chimpanzees from diffusion MRI using tractography and a set of predefined regions of interest to delineate fiber bundles. We propose here a novel atlas of the deep white matter connectivity in chimpanzees, established using diffusion MRI-based tractography and advanced fiber clustering techniques.Materials and methods
Chimpanzee cohort - A cohort of 39 chimpanzees including 23 females and 16 males aged from 9 to 25 years old stemming from the National Yerkes Primate Research Center (NYPRC, Atlanta) was studied in the frame of an imaging protocol approved by the Local Animal Welfare Committee.Imaging protocol - All individuals were scanned using a Siemens Tim Trio 3T MRI system with a birdcage coil with an imaging protocol including a 0.625mm isotropic MPRAGE T1-weighted scan and a series of 5 1.9mm isotropic diffusion MRI (dMRI) scans using a single-short 2D PGSE sequence (TE/TR=86ms/6s, RBW=1563Hz/pixel, matrix 128x128, FOV=243.2mm) at b=1000s/mm² along 60 diffusion directions.
Pre/Post-processing - After correcting for imaging artifacts7 and matching all individuals to the Juna.Chimp template8 using diffeomorphic registration9, dMRI was used to compute analytical Q-ball ODF maps10 (SH order 6, Laplace-Beltrami regularization factor of 0.006) from which a whole brain regularized deterministic tractography was launched (1 seed/voxel, forward step 0.4mm, aperture angle 30°) to obtain individual tractograms 11. A fiber clustering algorithm was then applied to the set of tractograms following the approach of Guevara et al4, relying on a two-step strategy starting with a first intra-subject hierarchical fiber clustering of connectograms relying on a hemisphere-based, length-based, and brain parcels-based partition of fibers to obtain individual clusters represented by their centroid, followed by a second cross-subject density-based spatial clustering (DBSCAN)12 of the individual centroid maps using a corrected pairwise maximum Euclidean distance between centroids.
The parameters were optimized in order to get an optimal number of clusters, yielding a normalization factor of 26, an affinity maximum distance of 18mm, an affinity maximum distance after exclusion of 4mm, and an affinity variance of 140625mm². A last step consisted in the identification of deep white matter bundles through the selection of cross-subject clusters from well-identified crossing points for each fascicle.
Results
39 individual whole brain tractograms were obtained, including a mean of 1503922 ± 158933 fibers. The intra-subject fiber clustering step performed on all fibers provided 5400 ± 747 clusters for the left hemisphere and 5473 ± 737 clusters for the right hemisphere at the individual scale, being slightly higher for the right hemisphere (see the processing on Figure 1.).The major long white matter fascicles that were found are (see Figure 2.) : The anterior and posterior thalamic radiations, the arcuate fasciculus, the cortico-spinal fasciculus, the cingulum, the uncinate fasciculus, the superior longitudinal fasciculus and the fornix. In comparison with the atlas from Guevara et al4, we can notice that all principle fascicles are present, with noticeable differences with respect to the human atlas : the arcuate fasciculus is less developed with less cortical projections confirming the observations of Rilling et al.13 and putatively being related to a less developed language function; the cortico-spinal tract follows the same pattern of traversing regions as for humans, starting from the brainstem, passing through medulla and thalamus to end course in pre-central post-central gyrus; however, due to the quadrupedal posture of the chimpanzee, the orientation of the bundles is different than the one of humans, the bipedal posture making the bundle more anterior and vertical; the cingulum is also closely similar with a bundle running along the cingulate gyrus; similarly, the uncinate fasciculus connects the lower part of the frontal lobe to the superior-anterior temporal lobe; the thalamic radiations and the fornix were also detected; the longitudinal fasciculus is also clearly delimited and connects the frontal lobe to the occipital lobe.
Conclusion
Following the first atlas of the chimpanzee brain structural connectivity recently published by Bryant et al6, we have proposed a novel chimp structural connectivity atlas relying on a different processing pipeline involving advanced fiber clustering techniques which replace the definition of regions of interest corresponding to crossroads of bundles that may depict important inter-subject variability by the use of elaborated metrics between fibers. This atlas now allows to automatically label the tractogram of any new subject without requiring the manual definition of regions of interest, thus providing an efficient tool to further study the differences of structural connectivity in chimps (sex or age differences) as well as its singularity with respect to humans.Acknowledgements
This work was partially funded by the Blaise Pascal Chair from Région Ile de France and University Paris-Saclay to W. Hopkins.References
- D. Goldman et al., PNAS, 1987 ; 84(10):3307‑11
- X. Li et al., Brain Structure and Function, 2017 ; 10.1007/s00429-016-1329-3
- W. D. Hopkins et al., Behav Brain Res, 2010 ; 10.1159/000319010
- P. Guevara et al., Neuroimage, 2012 ; 61(4), 1083-1099
- N. Labra et al., MICCAI, 2019 ; 10.1007/978-3-030-05831-9_25
- K.L Bryant et al., bioRxiv, 2020 ; 10.1101/2020.01.24.918516
- J.L.R. Andersson et al., NeuroImage, 2003 ; 20(2):870-888
- S. Vickery et al. bioRxiv, 2020 ; 10.1101/2020.04.20.04
- B.B. Avants et al.,NeuroImage, 2012 ; 54(3), pp.2033–2044
- M. Descoteaux et al., MRM, 2007 ; 58(3), 497-510
- M. Perrin et al., Inf Process Med Imaging, 2005 ; 10.1007/11505730_5
- M. Ester et al., KDD, 1996 ; 10.5555/3001460.3001507
- J.K. Rilling et al., Nature Neuroscience, 2008 ; 10.1038/nn2072
Figures
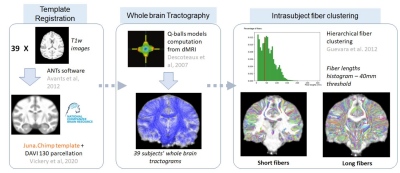
Figure 1. Steps allowing the obtaining of the long white matter bundles intra-subject clusters including a registration of the different anatomical and diffusion images to the template, a whole brain deterministic tractography, leading to an intra-subject fiber clustering.
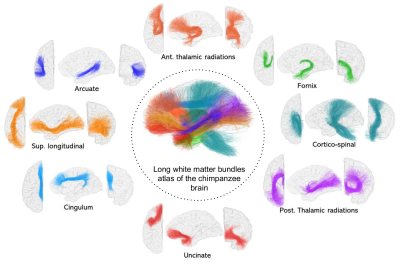
Figure 2. Left hemisphere principal long fascicles atlas of the chimpanzee brain. Views : Left- coronal, middle - sagital, right - frontal. Abbreviations : Sup. : Superior, Post : Posterior, Ant : Anterior