2568
Regional brain MRI features reveal the histological alterations in chronic pain: an MRI-based cell imaging study1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, shanghai, China, 2Human Phenome Institute, Fudan University, Shanghai, China, Shanghai, China
Synopsis
In our study, we used the ultra-high field magnetic imaging to show the brain functional and structure has changed in the rats of chronic pain model. then we also find the expression levels of the protein marker p-Erk has changed in some brain region, including insula, NAc, S1 and so on, and insula has been shown more significant activation. This results are same to the MRI functional data analysis. Finally, we hope to combine MRI and cell imaging technique could to verify the importance of insula in non-human chronic pain.
Introduction
Previous studies have proved that chronic pain was associated with brain function and structural changes, such as insula, nucleus accumbens1,2. However, whether these regions associated with chronic pain in the non-human subjects was unknown. Accordingly, we used the ultra-high field magnetic imaging to investigated the brain functional and microstructure change in the chronic pain model rat. Further, to validate the change in the cell level, we investigated the change of p-Erk (Mitogen-activated protein kinase) expression levels in the regions with functional change. p-Erk as a protein marker reveals the short-time cell activation state by external stimulation3. The results firstly identified the rat may share a similar brain mechanism of chronic with human. We hope the MRI and cell imaging study could contribute to highlight the importance of insula in non-human chronic pain.Model construction and biochemistry experiment
Chronic construction pain is performed under anesthesia, with the sciatic nerve on one side exposed by making a skin incision, and cutting through the connective tissue between the gluteus superficialis and biceps femoris muscles. Tow chromic gut ligatures are tied loosely around the sciatic nerve at 1 mm intervals, to just occlude but not arrest epineural blood flow. The wound is closed with sutures in the muscle and staples in the skin. After inducing pain stimulation, rats underwent rapid perfusion and PFA fixation, then underwent the frozen slice of the rat brain, and incubation with the immunoflouresence antibody (p-Erk). Finally, using confocal to perform the immunohistochemical image. In our study, three CCI rats and three healthy controls were used for further analysis. MRI scans were performed at two-time points, including two days before modeling and seven days after modeling. The histological analysis was performed on each rat after MRI scanning.MRI acquisition and Data processing
MRI experiments were performed in a BioSpec 500MHz 11.7 T system at the ZhangJiang Imaging Center of Fudan University. Functional data were acquired with single-shot echo-planar imaging (EPI) images (TR/TE 2000 ms / 11.83 ms, matrix 128 × 128 x 40, resolution: 0.5 mm, 220 time points). Diffusion data were acquired with spin-echo EPI imaging (TR/TE: 6000ms/17.4ms, matrix 96 × 96 x 50, resolution: 0.2 x 0.2 x 0.4 mm). 30 directions with b = 1000 s/mm2 and 5 unweighted b = 0 volumes. The rats were anesthetized and stabilized in the central area of the magnetic field with a volume coil. The rat brain was extracted manually. For functional data, the preprocessing pipeline including slice timing, realign, and normalized to a rat brain template4. The Alff of each rat was extracted for group analysis. For diffusion data, the data were corrected for eddy current and head motion, and the FA map was then fitted for each rat. The p-value was set at voxel-wise p<0.05 and clusters less than twenty voxels were excluded.Results
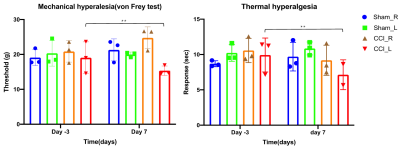
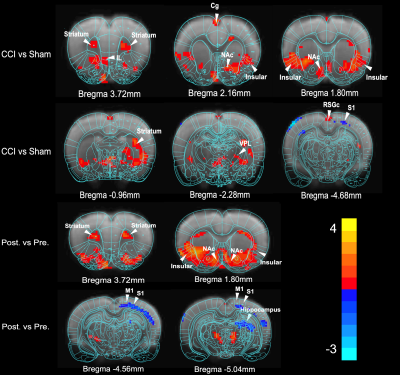
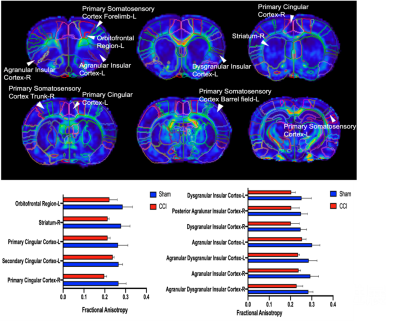
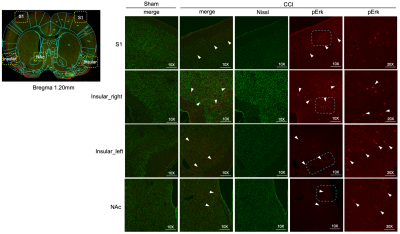
Behavior experiments showed that the CCI rats were more sensitive to the thermal and von Frey (mechanical pain), which suggested the model successfully constructed with chronic pain. The results showed that the CCI rats significant reduced ALFF in the bilateral striatal striatum and insula when compared to the shams controls. The comparison between pre-CCI and post-CCI rats showed that the Alff in the bilateral insula significantly increased in the post-CCI rats, while the Alff in the motor cortex was reduced for post-CCI rats. Fig.2 showed that the regional FA differences in the post-CCI rats and shams control. The results of the immunohistochemical showed that the same region of the significant difference in Alff comparison has also been activated (i.e. The dotted-line box and white arrow in the pErk column).Discussion
Prior study has suggested the insula was more related to acute pain rather than chronic pain in animal model5. Our animal study indicated that the insula was also involved in chronic pain. The Alff change in the bilateral insula suggested the brain spontaneous activity was increased, which suggested the chronic pain enhanced the sensitivity of sensory input. However, the lower FA in the insula also indicated the chronic may damage the microstructure integrity of insula. Our findings advance the understanding of insula in the chronic pain rat model. Firstly, the rat chronic pain may share a similar mechanism to human. Secondly, the rat model would also contribute to investigating the mechanism of chronic pain.Acknowledgements
References
1. Moore RA, et. al. Duloxetine use in chronic painful conditions--individual patient data responder analysis. Eur J Pain 2014, 18: 67-75.
2. Da Silva JT, Seminowicz DA. Neuroimaging of pain in animal models: a review of recent literature. Pain Rep 2019, 4: e732.
3. Haghparast A, et. al. Changes in the levels of p-ERK, p-CREB, and c-fos in rat mesocorticolimbic dopaminergic system after morphine-induced conditioned place preference: the role of acute and subchronic stress. Cell Mol Neurobiol. 2014 Mar;34(2):277-88.
4. Barrière DA, et. al. The SIGMA rat brain templates and atlases for multimodal MRI data analysis and visualization. Nat Commun. 2019 Dec 13;10(1):5699.
5. Bushnell MC, et. Al. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013 Jul; 14(7):502-11
Figures