2564
In vivo Fixel-Based Analysis of diffusion MRI in manifested Huntington’s disease in the zQ175 HD model1Bio-Imaging Lab, university of Antwerp, Antwerp, Belgium, 2Vision Lab, university of Antwerp, Antwerp, Belgium, 3CHDI foundation, Princeton, NJ, United States
Synopsis
Huntington’s disease (HD) is a debilitating neurodegenerative disease that affects the motor and cognitive abilities of patients. Diffusion-weighted imaging is often used in clinical evaluation of neurodegenerative disorders and recently a fixel-based analysis method was developed to analyse diffusion data. We implemented a multi-shell diffusion weighted acquisition and analysis in the zQ175 HD model. The results of the whole-brain and region-based diffusion tensor, diffusion kurtosis and fixel-based analysis indicate the presence of micro- and macrostructural differences in the zQ175 HD model in overall white matter fibers that can be attributed to significant changes in many fiber populations throughout the brain.
Introduction
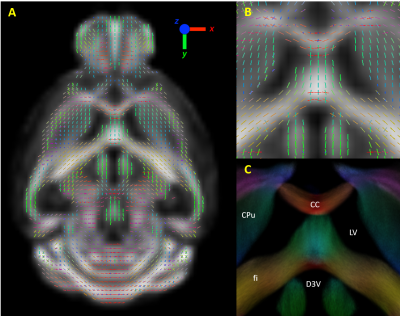
Huntington’s disease is a progressive genetic neurological disease that affects the motor and cognitive abilities of patients.1 Even though it is possible to test from birth whether a patient will develop HD, biomarkers are still crucial to distinguish different stages of the disease and to test potential HD treatments. Diffusion MRI and the commonly applied diffusion tensor imaging (DTI) analysis have already demonstrated the presence of structural deficits in human HD patients and animal models compared to healthy controls.2 Recently, a novel method of analyzing diffusion MRI data has been proposed which is the Fixel Based Analysis (FBA).3 This method uses constrained spherical deconvolution4 to extract different fiber orientations, and generate multiple fiber elements (fixels, fig 1), which contain detailed information on the macrostructure (fiber cross-section) and microstructure (density) of fiber tracts. In this study, we investigated (1) whether structural changes occur in the brain of zQ175 HD mice and (2) whether FBA could provide a more sensitive biomarker than the commonly used DTI/DKI analysis.Methods
Nine heterozygous zQ175 HD mice and nine healthy control littermates were investigated at 11 months of age (moderate manifest stage) using multi-shell diffusion MRI. Animals were anesthetized with 1-2% isoflurane in a mixture of N2 and O2 during handling and image acquisition. Temperature and breathing rate were monitored and maintained at (37.0 ± 0.1) oC and 90-120 breaths per minute by adjusting the concentration of isoflurane. Diffusion imaging was performed on a 7T Pharmascan 70/16 USR horizontal MR system (Bruker, Germany) using a multi-shell (b= 700, 1200 and 2800 s/mm2; δ= 4ms, Δ= 12ms) two-shot spin echo EPI sequence with 60 unique diffusion directions across each shell (TE/TR= 23/7000ms). Per scan, 3 b0 images were acquired. Each scan contained 16 slices (400mm slice thickness) with an in-plane resolution of (200x200)mm2 and a matrix size of [108x100]. Images were pre-processed using Gibbs ringing correction and denoising (MRtrix3.0),4 motion- and distortion correction (FSL6.0), and bias-field correction (ANTs). Voxel-level modelling was performed using multi-tissue constrained spherical deconvolution5 as available in MRtrix3.0. A multi-channel study-specific population template of the WM and GM compartment was built using iterative nonlinear registration.6,7 Data was statistically compared in Mrtrix3.0 using a fixel-wise two sample t-test. A similar processing pipeline was used before fitting the diffusion data to the DKI model, from which diffusion metrics (FA, MD, RD, AD) and kurtosis metrics (MK, RK, AK) were extracted. A voxel-wise two sample t-test was performed on the diffusion/kurtosis maps using FSL6.0 randomise,8 which performs nonparametric permutation testing. All data reported are statistically significant for PFWE<0.05. Regions of interest were delineated based on the outcome of the FBA, from which mean values for each metric were extracted and statistically compared using an unpaired t-test, followed by False Discovery Rate correction to correct for false positives.Results
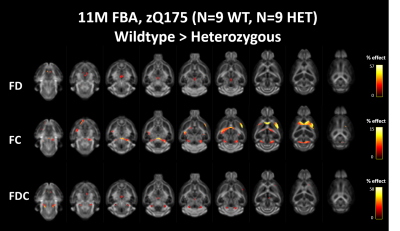
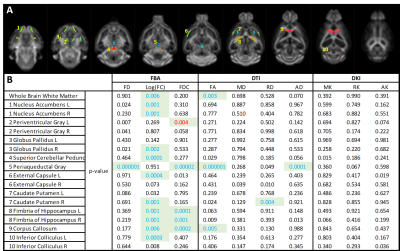
Both diffusion tensor metrics and Fixel-based analysis reveal significant structural differences at a manifest stage of HD in the zQ175 mouse model (fig 2&3b). No significant differences could be found in the diffusion kurtosis metrics (fig 3b). While DTI analysis was able to pick up significant differences between Heterozygous animals and healthy littermates, these differences were spatially less extensive than those observed in the FBA. As expected from a neurodegenerative disease, most significant differences were caused by a decrease of fiber density and cross-section in zQ175 HET animals. Fiber-density, a measure of fiber bundle axon density, is significantly lower in zQ175 HET animals in periaqueductal gray (p<0.001). Fiber cross-section, a measure of fiber bundle diameter, shows the most extensive differences (fig 2,3b). zQ175 HET animals have significantly reduced fiber cross-sections in the cerebellum related fiber tracts (cerebellar peduncles, inferior colliculus, p<0.001), medial forebrain bundles (fimbria, p=0.001) and lateral forebrain bundle system (corpus callosum, external capsule, p<0.006). The combination metric, the fiber density x cross-section, was lower in zQ175 HET animals in the cerebellar peduncles, periaqueductal gray (p<0.001) and medial forebrain bundle system (fimbria, p<0.001) (fig 3b). zQ175 HET animals have lower FA in overall white matter (p=0.003), the corpus callosum (p=0.005) and the periaqueductal gray (p<0.001). RD is significantly reduced in caudate putamen and AD is lower in the periaqueductal gray (fig 3b).Discussion & Conclusion
This study reports the first ever diffusion investigation of the zQ175 model. We have shown widespread structural differences in the zQ175 model. These regions include structures of the medial and lateral forebrain bundles that have previously been linked to HD2, such as the corpus callosum and the cerebellar peduncles. The corpus callosum is frequently reported to be affected in HD2, which were picked up with the FC metric in the FBA. This finding in combination with the extend of the differences suggest that FBA could be a more precise modeling approach than the commonly used DTI. Possible explanations for these structural deficits could be changes in myelination of axons or differences in the number/volume of axons that are present in these bundles. Further longitudinal investigation is on-going with a larger sample size to elucidate the progression of these differences, starting from an early timepoint. Histological validation of data will be performed to elucidate the mechanism(s) responsible for these structural deficits.Acknowledgements
This research was supported by the CHDI Foundation, Inc.References
1. Bates, G. P. et al. Huntington disease. Nat. Rev. Dis. Prim. 1, 1–21 (2015).
2. Estevez-Fraga, C., Scahill, R., Rees, G., Tabrizi, S. J. & Gregory, S. Diffusion imaging in Huntington’s disease: comprehensive review. J. Neurol. Neurosurg. Psychiatry 92, 62-69 (2020)
3. Raffelt, D. A. et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144, 58–73 (2017).
4. Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202, 116137 (2019).5.
5. Jeurissen, B., Tournier, J. D., Dhollander, T., Connelly, A. & Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103, 411-426 (2014)
6. Raffelt, D., Tournier, J. D., Fripp, J., Crozier, S., Connelly, A. & Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 56, 1171-1180 (2011)
7. Pietsch, M. et al. A framework for multi-component analysis of diffusion MRI data over the neonatal period. Neuroimage 186, 321-3377 (2019)
8. Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397 (2014).
Figures