2563
Alteration of basal ganglia thalamo-cortical loop in hemiparkinsonian mouse model1Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2Inserm UMR-S 1270, Paris, France, 3Sciences and Technology Faculty, Sorbonne Universite, Paris, France, 4Institut du Fer à Moulin, Paris, France, 5NeuroSpin/CEA-Saclay, Gif-sur-Yvette, France, 6Inserm, UMR1266, Paris, France, 7Sorbonne Universite, Paris, France
Synopsis
Despite a few studies, our knowledge of functional connectivity in the basal ganglia-thalamo-cortical loop remains incomplete in Parkinson’s disease mouse model. Here, we investigated alterations of functional connectivity and white matter structure in a hemi-parkinsonian mouse model. We found that the fractional anisotropy significantly decreased in the lesioned side in the subthalamic nucleus, such as medio-dorsal nucleus and centromedian nucleus of thalamus. The functional connectivity in the ipsilateral thalamic nuclei was also decreased in hemiparkinsonian mice. These results indicate that ipsilateral thalamic nuclei are key regions for functional and structural alterations in basal ganglia-thalamo-cortical loop in hemiparkinsonian mice.
Introduction
The basal ganglia-thalamo-cortical loop, which includes the striatum, the globus pallidus and the substantia nigra, is a key dysfunctional network in many motor disorders like Parkinson’s disease (PD)1. The resting state fMRI has the advantage to study in vivo the functional connectivity between anatomically separated regions in the brain. Despite a few studies using this approach, alterations in functional connectivity in the basal ganglia-thalamo-cortical loop have not been studied in detail in the PD mouse model2. In a previous study, we developed resting state fMRI in the mouse after chemo-genetic stimulation of a specific neuronal population of the striatum, the entry structure of basal ganglia-thalamo-cortical loop3. In the present study, we used this approach in hemiparkinsonian mice to investigate the altered basal ganglia-thalamo-cortical loop.Methods
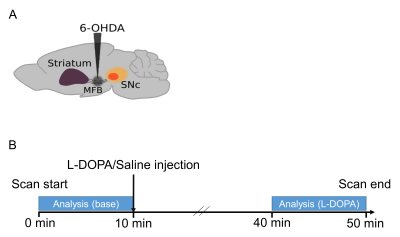
Animals and surgeryUnder anesthesia with ketamine-xylazine, twenty-one male C57BL/6 mice (20-30 g) were injected with 6-OHDA (lesioned, n=13) or saline (sham-operated, n = 8) into the median forebrain bundle (AP, -1.2 mm; L, +1.1 mm; DV, -5 mm from Bregma) (Fig.1A). The mice were allowed to recover for more than 4 weeks before fMRI.
fMRI experiment
fMRI acquisition was conducted at Bruker 11.7 T with a cryoprobe. fMRI images were acquired using a gradient-echo EPI sequence, TR/TE = 2,000/15 ms, spatial resolution = 100 x 100 x 500 µm3 / pixel, 15 slices, for 50 min (1500 volumes). The protocol outline is shown in Fig. 1B. The L-DOPA and benserazide (10 and 12 mg/kg body weight, respectively) or saline were injected intraperitoneally 10 min after the start of scanning. Scanning continued for up to 40 min following the injection. Respiration and body temperature were maintained at a rate of 80/min and 37 °C, respectively. Anatomical images were acquired for spatial normalization using multi-slice rapid acquisition with relaxation enhancement (RARE) following parameters: TE/TE = 2,500/48 ms, same FOV with high resolution (100 x 100 x 500 µm3 / pixel) and RARE factor = 8.
fMRI processing
The images were obtained after slice timing correction and spatial correction (realignment and normalization) using SPM12. Then the averaged signals in the CSF, the white matter and the head motion parameters are used as nuisance signals. Detrending and temporal filtering (0.01 – 0.1 Hz) were then performed. For correlation analysis, 36 regions of interest (ROIs) including the striatum, the globus pallidus, the substantia nigra and the thalamic nuclei were created using the Allen Brain Atlas. Time-course within each ROI was extracted and Pearson’s correlation coefficients with the other ROIs were calculated.
Diffusion tensor imaging (DTI)
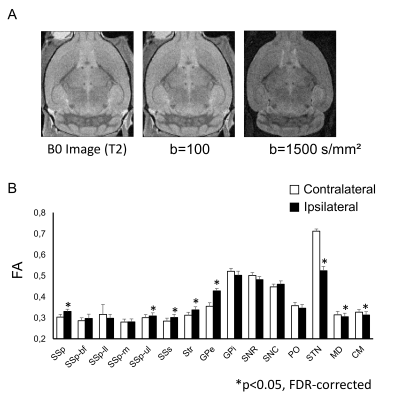
DTI Images were acquired after brain fixation in paraformaldehyde (4 %). We used a 17.2 T Bruker MRI system with a volume coil of 9 mm in diameter. A diffusion-weighted spin echo sequence with the following parameters was used: time of repetition, 9500 ms; echo time, 17 ms; resolution, 103 µm × 103 µm × 150 µm/pixel); δ = 3 ms; Δ = 10 ms. Symmetric diffusion gradients were applied with b = 100 and b = 1000 s/mm² in 21 non-collinear directions and b = 0 s/mm² for the reference (Fig. 2A). Fractional anisotropy (FA) was calculated using DSI Studio.
Results
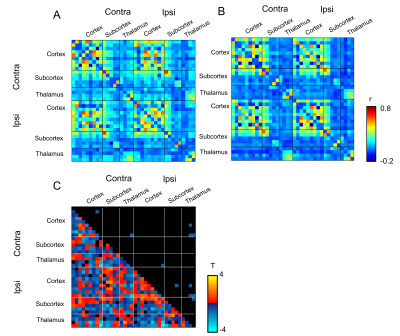
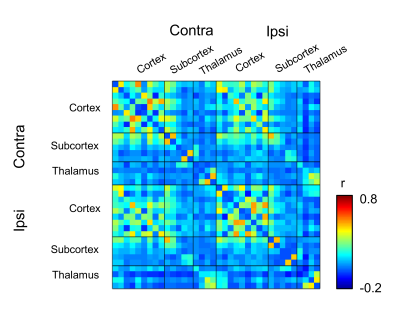
FA values in the ipsilateral subthalamic nucleus as well as medial dorsal and centromedian nuclei of the thalamus were significantly lower than those in contralateral side (Fig. 2B, *p < 0.05). In contrast, FA values in the ipsilateral somatosensory cortex, striatum and external globus pallidus were higher than those in the contralateral side. We then investigated the differences in the functional connectivity between lesioned and sham groups during 10 min before injection of L-DOPA (Figs. 3A and 3B). The correlation coefficients between contralateral thalamic nuclei and several ipsilateral/contralateral regions were significantly lower in lesioned mice than in sham mice (Fig. 3C, *p < 0.05, NBS-corrected). These low correlation coefficients in lesioned mice were partially recovered between 30 and 40 min after the injection of L-DOPA (Fig. 4) rather than those between 0 and10 min before injection (Fig. 3B). We confirmed that there was no significant change in the functional connectivity following saline injection.Discussion
In the present study, we show significant alterations of the white matter ultrastructure and the functional connectivity in hemiparkinsonian mice. Both FA and functional connectivity of the ipsilateral thalamic nuclei were decreased, indicating that functional abnormality of these regions could be caused by structural alterations in lesioned mice. However, a functional connectivity was partially recovered following the injection of L-DOPA, consistent with the attenuation of motor deficits in lesioned mice.Acknowledgements
No acknowledgement found.References
1. Albin, R.L, Young A.B., Penney J.B., The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18: 63–64
2. Monnot C, Zhang X, Nikkhou-Aski S, Damberg P, Svenningsson P, Asymmetric dopaminergic degeneration and levodopa alter functional corticostriatal connectivity bilaterally in experimental parkinsonism. Exp Neurol. 2017;292: 11-20.
3. Nakamura Y, Nakamura Y, Pelosi A, Djemai B, Debacker C, Hervé D, Girault JA, Tsurugizawa T, fMRI detects bilateral brain network activation following unilateral chemogenetic activation of direct striatal projection neurons, NeuroImage. 2020 Oct 15;220:117079.
Figures