2556
Longitudinal brain network changes in the GAERS rat model of absence epilepsy1Translational Research Imaging Center Clinic for Radiology, University of Münster, Münster, Germany, 2Physiology I, University of Münster, Münster, Germany
Synopsis
Resting-state fMRI under Isoflurane was performed to characterize brain networks in 4 and 8 month old Genetic Absence Epilepsy Rats from Strasbourg (GAERS) and in non-epileptic controls (NEC). Graph theoretical analysis identified major differences in intra-thalamic connectivity with age and compared to NEC, indicating that thalamus is strongly involved and probably modulated by frequently occurring seizures. In contrast, in NEC, brain networks did not change considerably in the age range studied.
Introduction
Absence epilepsy is a non-convulsive type of childhood epilepsy characterized by seizures occurring several hundred times per day accompanied by impaired consciousness and behavioral arrest. The Genetic Absence Epilepsy Rats from Strasbourg (GAERS) resembles many features of the human disease and is therefore well suited to investigate the epileptic brain network in comparison to non-epileptic controls (NEC)1. Here we performed resting-state (rs-) fMRI in GAERS and NEC at 4 and 8 months of age under low dose Isoflurane anesthesia and used graph theory analysis2 to identify potential long-term brain network alterations.Methods
rs-fMRI with GE-EPI (TR 1 s, TE 18 ms, resolution 0.32x0.35 mm2, 1.2 mm slices, 12 slices, 30 min scan time) at 9.4 T (Bruker Biospec) was performed in GAERS (n=12) and NEC (n=12) at 4 and 8 months of age under 1-1.2% isoflurane anesthesia. Data was preprocessed (realign & reslice) with SPM. The first 15 minutes of raw data were used. Continuous 15 min epochs with stable translation and rotation parameters were selected from 30 min data sets, in case any shift in rat head position was detected. The resulting data was smoothed, masked and registered to an anatomical atlas template based on the Paxinos and Watson rat brain atlas3 with MagnAN (Biocom, Germany)4 a MR image analysis software based on IDL (© 2020 Harris Geospatial Solutions, Inc). Cross-correlation matrices of regional time courses were calculated and averaged. We determined global (clustering coefficient, path length, small world index) and local node (strength, path length, hub score) parameters by graph theory analysis. Gephi5 was used to calculate and display community structure of brain networks. Network-based-statistics (NBS)6 was applied to compare connections between brain regions at α=0.05.Results
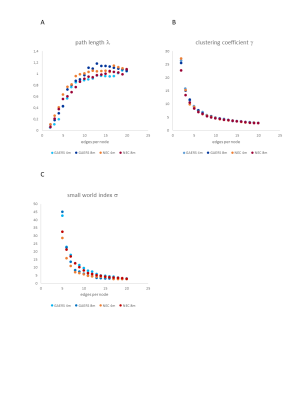
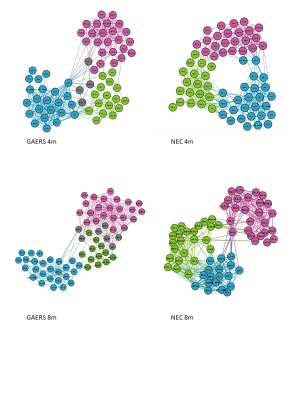
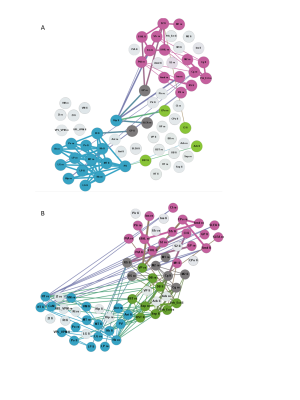
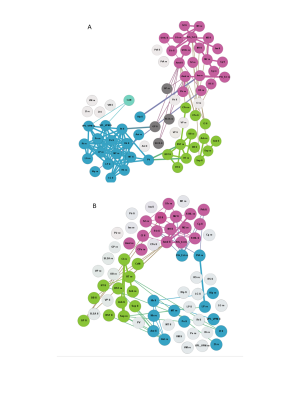
The global network parameters were preserved independent of rat strain and age (fig. 1). Functional groups gathered in largely similar communities in GAERS and NEC at both time points (fig. 2). In NEC 3 communities were observed, one large community comprising mostly cortical areas, one containing primarily thalamic structures and another one with limbic and basal ganglia regions. In GAERS, motor cortex areas formed a fourth community together with retrosplenial cortex and cingulate cortex at both time points. At 4 months, many statistically stronger intra-thalamic and intra-cortical connections appeared in GAERS (fig. 3A). The left hippocampus appeared prominent with statistically strong connections to cortical areas (insular cortex, primary somatosensory cortex), basal ganglia areas (globus pallidus, striatum) and areas of the limbic system (sublenticular extended amygdala). In NEC more, significantly stronger connections between cortical and thalamic areas indicated more exchange between communities (fig. 3B). In NEC, also connections between left and right hippocampus were strong. At 8 months, statistically stronger intra-cortical connections were found in GAERS, while in NEC stronger connections within the thalamic community and stronger connections between thalamic, cortical and limbic areas were observed. When comparing GAERS at different ages (fig. 4A), the high number of statistically stronger intra-thalamic and intra-cortical connections stood out in younger rats. There were only minor differences between connections in NEC at 4 and 8 months (fig. 4B).Discussion
Preserved global node parameters and community structure suggest that both, GAERS and NEC, have efficiently working networks. rs-fMRI data were acquired under Isoflurane anesthesia, which suppresses seizure activity. We kept anesthetic dose and duration as low and as short as possible and identified differences between groups which may reflect long term changes due to frequently occurring seizures. In particular the cortical regions were much better interconnected in GAERS, but segregated from the remaining network at 4 and 8 months of age. Major differences in intra-thalamic connectivity with age and compared to NEC indicate that thalamus is strongly affected and probably modulated by frequently occurring seizures. In contrast, in NEC, brain networks do not change considerably in the age range studied.Conclusion
Despite suppressive effects of Isoflurane on seizure activity and general brain activity, graph theoretical analysis of rs-fMRI data revealed age-dependent changes in GAERS. Since NEC showed largely similar networks at both time points, a pronounced long-term effect on brain networks resulting from seizures appears to be present.Acknowledgements
No acknowledgement found.References
1. Depaulis, A., David, O., & Charpier, S. (2016). The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. Journal of Neuroscience Methods, 260, 159–174. https://doi.org/10.1016/j.jneumeth.2015.05.022
2. Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. https://doi.org/10.1016/j.neuroimage.2009.10.003
3. Paxinos, George; Watson, C. R. (2007). The rat brain in stereotactic coordinates. London Academic Press.
4. Kreitz, S., Alonso, B. de C., Uder, M., & Hess, A. (2018). A new analysis of resting state connectivity and graph theory reveals distinctive short-term modulations due to whisker stimulation in rats. Frontiers in Neuroscience, 12(MAY), 1–19. https://doi.org/10.3389/fnins.2018.00334
5. Bastian M., Heymann S., J. M. (2009). Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media.
6. Zalesky, A., Fornito, A., & Bullmore, E. T. (2010). Network-based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. https://doi.org/10.1016/j.neuroimage.2010.06.041
Figures