2555
Can Diffusion Weighted MRI Predict the Response Prior to Neoadjuvant Chemotherapy in Patients with Muscular Invasive Bladder Cancer?1Department of Imaging Diagnosis, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College, BeiJing, China
Synopsis
Neoadjuvant chemotherapy(NAC) becomes the standard treatment for muscular invasive bladder cancer (MIBC), some patients do not respond to NAC. So the purpose of this study was to evaluate the potential of diffusion-weighted (DW) magnetic resonance imaging (MRI) with an ADC map in the prediction of response to NAC in patients with MIBC. In our study, all the sixty-two MIBC patients have underwent DW-MRI before and after NAC. After NAC, patients were classified into responders or non-responders by the Response Evaluation Criteria in Solid Tumors. However, there was no statistically significant difference in ADC between responders and non-responders before NAC.
Background
Bladder cancer is the 10th most common Malignant tumor worldwide1. Neoadjuvant chemotherapy provides a survival benefit2 and becomes the standard treatment for muscular invasive bladder cancer (MIBC)3. However, 50 to 60 percent of patients do not response to neoadjuvant chemotherapy4. So early and accurate identification of non-responders and changing treatment strategies can avoid side effects and surgical delays.Objectives
To investigate the performance of diffusion weighted (DW) MRI with an apparent diffusion coefficient (ADC) map for predicting the neoadjuvant chemotherapy (NAC) response in patients with MIBC.Patients and methods
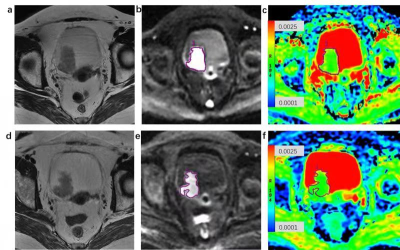
Sixty-two patients with clinical T2-4a MIBC underwent DW-MRI before and after NAC followed by surgery. Treatment response was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST,version1.1). Complete response and partial response patients were estimated as responders. Patients with progressive disease and stable disease were defined as non-responders. Tumor ADCs was measured by two radiologists. Interobserver variability was analyzed by calculating intraclass correlation coefficient (ICC). The pre-and post-NAC ADCs and percentage increases in ADC after NAC were compared between responders and non-responders by Mann–Whitney U-test.Results
After NAC, 34 (55%) patients were classified as responders, and 28 (45%) were classified as non-responders. There was no difference in the pre-NAC ADC between responders and non-responders(p=0.983). However, the post-NAC ADC of responders([1.62(±0.30)] ×10-3 mm2/s) was significantly higher than that of non-responders([1.29(±0.21)] ×10-3 mm2/s). The mean percentage increases in ADC were significantly different between responders (33.0%±31.7) and non-responders (5.5%±29.9) (p < 0.001). ICCs were good for pre- and post-NAC ADC measurements (0.969 [95% CI: 0.949, 0.981]and 0.973 [95% CI: 0.955, 0.984], respectively).Conclusion
The baseline ADC value before treatment could not predict tumor response to NAC in patients with MIBC. After NAC treatment, the ADC value of responders increased significantly.Acknowledgements
Contributed guarantor: Yan Chen ; Contributed ethical approval: Institutional Review Board of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College;Contributed statistical analysis:Xiaojuan Xu;Contributed to performing the experiments: Yichen Wang, Jin zhang, Lianyu zhang.References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R. L, et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68 (6): 394-424.
2. Yin M, Joshi M, Meijer R. P, et al. Neoadjuvant Chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016; 21 (6): 708-715.
3. Patel V. G, Oh W. K, Galsky, M. D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020.
4. Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011; 29 (16): 2171-2177.
Figures