2544
Intravoxel Incoherent Motion Imaging of Renal Cold Ischemia Reperfusion Injury in Rats1Tianjin First Center Hospital, Tianjin, China, 2First Central Hospital Institute, Tianjin Medical University, Tianjin, China, 3MR collaborations, Siemens Healthcare Ltd., Beijing, China, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
The study evaluated the use of Intravoxel Incoherent Motion (IVIM) imaging to detect dynamic changes in renal microvascular characteristics during cold ischemia-reperfusion injuries (CIRIs). As previous studies have investigated warm ischemia-reperfusion injuries, we aimed to assess MR diffusion imaging in a renal CIRI Sprague Dawley rat model. Results showed that IVIM imaging is a sensitive tool to monitor changes in renal functional characteristics.
Synopsis
The study evaluated the use of Intravoxel Incoherent Motion (IVIM) imaging to detect dynamic changes in renal microvascular characteristics during cold ischemia-reperfusion injuries (CIRIs). As previous studies have investigated warm ischemia-reperfusion injuries, we aimed to assess MR diffusion imaging in a renal CIRI Sprague Dawley rat model. Results showed that IVIM imaging is a sensitive tool to monitor changes in renal functional characteristics.Purpose and background
Cold ischemia-reperfusion injury (CIRI) is one of the most serious pitfalls in kidney transplantation and inevitably plays a critical role in renal transplantation [1]. CIRI increases the risk of acute renal injury[2], may develop into chronic kidney disease, and even cause delayed graft function (DGF) [3]. The effect of CIRI on the transplanted kidney is more prominent due to the longer cold preservation time of the donor kidney in the process of renal transplantation. Therefore, it is crucial to monitor CIRI physiological changes effectively, especially because renal CIRI is reversible in most cases and the survival rate of the transplanted kidney can be improved[4]. Intravoxel Incoherent Motion (IVIM) imaging can reflect dynamic microscopic changes in molecular water diffusion and microcirculation sensitively and repeatably. These features make IVIM a suitable noninvasive tool to detect renal injury. The aim of this study was to investigate the feasibility of IVIM DWI in the noninvasive assessment of a rat model in renal CIRI.Material and methods
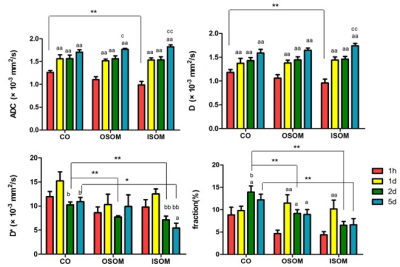
This study consisted of forty healthy male Sprague Dawley rats divided into two groups: the experimental group – left kidney cold ischemia-reperfusion model group (n=20); and the sham-operation group – right kidney resected and left kidney preserved group (n=20). Five rats from each group were randomly selected for IVIM imaging at four time points after surgery (1 hour, and 1, 2, and 5 days) on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with an 8-channel experimental animal coil (Shanghai Chenguang Medical Technology Co., Ltd). The IVIM images were obtained using single-shot diffusion-weighted echo-planar imaging with the following parameters: TR/TE=2300/74 ms,FOV=140×114 mm2, matrix=120×98, slice thickness=3.0 mm, and 10 b-values [0,10,20,30,50,100,200,300,500,800] s/mm2 obtained in three diffusion gradient directions. IVIM-derived parameters (pure molecular diffusion: D, pseudo-diffusion: D*, and the perfusion fraction: f were calculated using a prototype software (MR Body Diffusion Toolbox, Siemens Healthcare). Nine regions of interest (ROIs) were positioned in the cortex (CO), outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM) in the upper, middle, and inferior parts for each kidney. ROIs had areas of 1-2 mm2, and each ROI included 4-7 pixels. The quantitative parameter measurements including apparent diffusion coefficient (ADC), D, D*, and f were averaged for the final results. Blood was collected from the abdominal aorta to obtain blood urea nitrogen (BUN) and superoxide dismutase (SOD). Following imaging, the left kidney was removed for hematoxylin eosin (HE) staining to acquire the pathological scores. According to the Paller’s standard[5], higher scores represented more severe damage. The t-test of two independent samples was used to compare the differences of parameters of two groups at the same time point. ANOVA and least significant differences (LSD) were used to compare parameters of anatomical regions at different time points in each group. Pearson correlation analysis was used to evaluate the correlation between the imaging parameters and the kidney injury scores and biochemical indexes.Results
The detailed results of the sham-operation and experimental group were shown at the Fig. 3 and Fig. 4 respectively. The ADC and D were the lowest at 1 hour and elevated to the highest on day 5. On day 1, the ADC and D of the ISOM and OSOM in the experimental group were significantly lower than those in the sham operated group (P < 0.05). On day 2, the D* and f values of the OSOM in the experimental group were significantly lower than those in the sham-operated group (both P < 0.05). There was no significant difference found in any quantitative parameters between the two groups at 1 hour and day 5. There was a moderate negative correlation between the f of the OSOM and the renal tubular injury score (r = -0.462, P < 0.01) in the both groups. The f of the OSOM in the experimental group was moderately negatively correlated with the pathological scores (r = -0.611, P <0.01). The D* of CO was positively correlated with urea nitrogen (r = 0.461, P < 0.01). In the experimental group, the f value of OSOM was negatively correlated with SOD (r = -0.455, P < 0.05).Discussion
The results presented here demonstrate that quantitative ADC, D, D*, and f values can be used to evaluate water molecule activation at the level of microcirculation perfusion of the kidney. These parameters markedly changed over time. Significant difference between the experimental and sham-operation groups indicate that CIRI may influence the kidney most on day 1. Pathological scores and experiment indicators were moderately correlated with the imaging parameters, suggesting IVIM may be able to reflect microscopic physiological changes.Conclusion
In conclusion, IVIM has great potential in monitoring renal CIRI progression. Measurements of diffusion anisotropy and apparent water diffusivity could provide structural and functional information about the kidneys following renal CIRI.Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China(81873888).References
[1]Kootstra G , Heurn E V . Non-heartbeating donation of kidneys for transplantation. Nature Clinical Practice Nephrology 2007;3:154-163.
[2] Hart A, Gustafson SK, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: early effects of the new kidney allocation system. Am J Transplant 2017,17: 543-564.
[3]Zhang W , Zhao J , Cao F , et al. Regulatory effect of immunosuppressive agents in mice with renal ischemia reperfusion injury. Experimental and therapeutic medicine 2018;16: 3584-3588.
[4] Francis A, Baynosa R. Ischemia-reperfusion injury and hyperbaric oxygen pathways: a review of cellular mechanisms. Diving Hyperb Med 2017;47:110-117.
[5]Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat . J Clin Invest,1984;74:1156-1164.
Figures