2539
Improving the robustness of pseudo-continuous arterial spin labeling for renal perfusion imaging1Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Pseudo-continuous arterial spin labeling (pCASL) has been the recommended ASL method for brain, and one of the two methods for kidneys. However, the reliability of labeling efficiency of pCASL is a major concern, especially in renal imaging due to the increased B0 inhomogeneities. In this study, we implemented and evaluated the optimized unbalanced pCASL gradient scheme generated by numerical simulation with both perfusion phantom and 4 healthy volunteers. The results showed that this optimized unbalanced pCASL gradient scheme was more robust to off-resonance than the corresponding balanced pCASL gradient scheme.
Introduction
Recent consensuses have identified pseudo-continuous arterial spin labeling (pCASL) as the labeling method of choice for ASL in both brain (1) and kidneys (2). However, one of the major concerns of pCASL is the reliability of labeling efficiency, especially in renal application. The increased off-resonance at the labeling plane, which often occurs near the lungs, decreases labeling efficiency and sometimes can lead to complete loss of perfusion signal (3). Recent studies have shown improved robustness of B0 inhomogeneities in brain by pCASL with unbalanced labeling scheme (4). Therefore, the goal of this study was to demonstrate the increased robustness of pCASL in renal applications using an optimized unbalanced gradient scheme, evaluated in both perfusion phantom and healthy volunteers.Methods
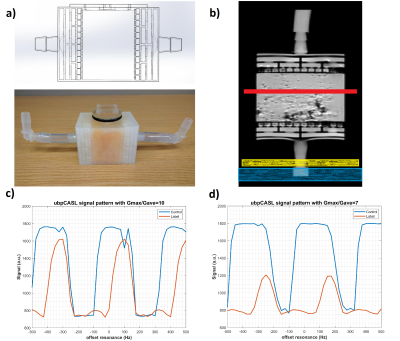
To optimize the labeling efficiency, numerical simulations of Bloch equations were performed to evaluate different unbalanced gradient schemes for both tortuous flow and off-resonance as previously described (4, 5). The robustness of pCASL sequence with the optimized unbalanced gradient scheme was tested both on a previously designed 3D printed perfusion phantom (6) and 4 healthy volunteers’ kidneys with IRB approval on a 3T MRI scanner (Ingenia, Philips Healthcare).Image Acquisition and Analysis: The off-resonance effects were approximated by adding extra phase offsets between RF pulses during the labeling. All pCASL images were reconstructed in MATLAB including k-space filtering and complex k-space subtraction.
For perfusion phantom: pCASL images were acquired in a single axial plane using single shot TSE with the following parameters: TR/TE = 6000/16 ms, FOV = 150x150 mm2, matrix = 64x63, acquired resolution = 2.34x2.38x3 mm3, echo spacing = 3.2 ms, ETL = 63, label duration = 1.8 s, post-label delay = 1.8 s, labeling RF interval = 1.2 ms, 1 repetition, 4 background suppression pulses and inflow saturation pulses. To compare the robustness of balanced and unbalanced gradient scheme at different off-resonance, 2D pCASL with 41 dynamics were performed for each phase offset ranging from -500 Hz to 500 Hz at 25 Hz increments. An unbalanced gradient scheme was also run with Gmax/Gave ratio of 10 and 7, with the Gave of 0.5 mT/m. The total acquisition time for each 2D pCASL with 41 dynamics was 8:58 minutes.
For healthy volunteers: All 2D pCASL images were acquired in a single coronal plane using single shot TSE scans with the following parameters: TR/TE = 6500/42 ms, FOV = 223x375 mm2, matrix = 88x150, acquired resolution = 2.53x2.50x10 mm3, reconstructed resolution = 0.59x0.59x10 mm3, echo spacing = 4.7 ms, ETL = 83, label duration = 1.5 s, post-label delay = 1.5 s, labeling RF interval = 1.2 ms, Gmax = 3.5 mT/m, Gave = 0.5 mT/m, 4 background suppression pulses and inflow saturation pulses. To compare the robustness of unbalanced and balanced gradient schemes to different off-resonance, 2D pCASL images were acquired with 4 signal averages at each phase offset ranging from -300 Hz to 300 Hz at 50 Hz increments (total 13 dynamics). The total acquisition time for 2D pCASL with 13 dynamics and 4 averages was 11:38 minutes. An additional set of images without extra off resonance was also acquired using the unbalanced and balanced gradient schemes with 16 signal averages at 3:24 minutes each. A separate M0 was acquired in 7 seconds with the same acquisition parameters for renal blood flow quantification.
Results
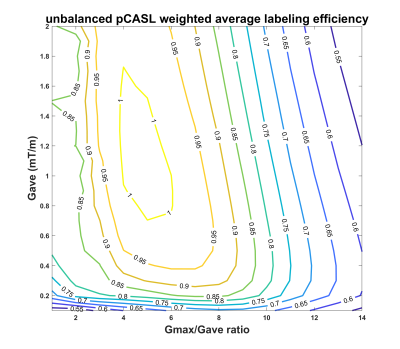
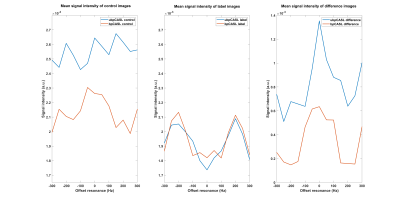
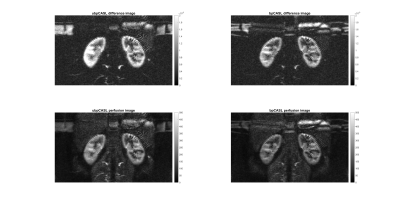
The numerical simulations demonstrated high labeling efficiency with the unbalanced pCASL gradient scheme with Gmax/Gave ratio between 5 to 7 and Gave greater than 0.4 mT/m (Fig. 1) across broad off-resonance (0-420 Hz) and blood velocity (0-123 cm/s). In perfusion phantom, the unbalanced pCASL with gradient ratio of 10 (Fig. 2c) had lower labeling efficiency than the unbalanced pCASL with gradient ratio of 7 (Fig. 2d), while the control signal remained intact. This demonstrated that unbalance pCASL with Gmax/Gave ratio of 7 will have higher signal difference between control and label images and are more robust to off-resonance. This behavior is also observed in normal volunteer’s kidneys, showing increased robustness to off-resonance with unbalanced pCASL (Fig. 3-5). Figure 4 interrogates the signal intensity and since the Gave in unbalanced pCASL control images is 0, it is more robust to off resonance giving higher signal intensity than balanced pCASL whose Gave in control is not equal to zero.Discussion and Conclusion
Numerical simulations suggest that high labeling efficiency can be achieved using unbalanced pCASL gradient scheme with Gmax/Gave ratio between 5 to 7 and Gave greather than 0.4 mT/m across a broad range of off-resonance and blood velocities. This optimized unbalanced pCASL gradient scheme (Gave = 0.5 mT/m, Gmax/Gave = 7) improves the robustness of pCASL labeling to off-resonance effects in both perfusion phantom and healthy volunteers’ kidneys.Acknowledgements
This work was partly supported by the NIH/NCI grant U01CA207091.References
1. Alsop, D.C., et al., MRM, 2015. 73(1): p. 102-16.
2. Nery, F., et al., MAGMA, 2020. 33: p. 141-161.
3. Echeverria-Chasco, R., et al., MRM, 2020. 85 (3): p. 1507-1521.
4. Zhao, L., et al., MRM, 2017. 78(4): p. 1342-1351.
5. Maccotta L., et al., NMR Biomed, 1997;10(4–5):216–221.
6. Greer, J.S., et al., ISMRM 2017:3805.
Figures