2537
Navigator-triggered kidney vessel architecture imaging1Department of Diagnostic and Interventional Radiology, Heidelberg University Hospital, Heidelberg, Germany, 2Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 3Department of Radiology, German Cancer Research Center, Heidelberg, Germany
Synopsis
Vessel architecture imaging (VAI) MRI is a a technique that noninvasively measures parameters who describe the structural heterogeneity of brain microvasculature. To apply VAI in kidney disease respiratory motion artifacts need to be compensated for. In this study, a navigator along the inferior-superior direction was inserted as training data at the beginning of the measurement and interleaved during imaging acquisition. Our preliminary results suggest that respiratory motion can be corrected accurately.
PURPOSE
Vessel architecture imaging (VAI) MRI is a useful technique for noninvasive measurement of topological and structural heterogeneity of the microvasculature1. This technique has been successfully applied in brain imaging. However, to apply VAI in abdominal imaging, respiratory motion is an additional problem which can cause motion artifacts. To fully track the required contrast agent bolus, a breath-hold of 35s is necessary. However, this is too long for many patients. Therefore, in this study, respiratory motion information is calculated from a projection signal during the kidney VAI is calculated for navigating the imaging slices. The slice positioning is changed in real-time based on the motion information.METHODS
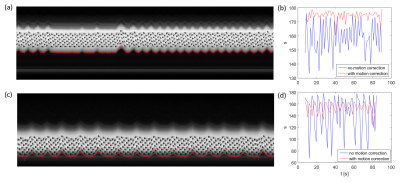
Measurements were performed using an 18-channel body and spine receive RF coil on a 1.5T Aera Siemens scanner (Siemens Healthcare, Erlangen, Germany). A single gradient-echo slice selection and projection readout at the location of the diaphragm along the inferior-superior (IS) direction is applied as a navigator. Navigator acquisition and fat suppression were inserted before each transverse imaging slice of the readouts of a dual gradient echo (GE)/spin echo (SE) 2D EPI, with 60 measurements including 4000 training navigators at the beginning of measurements obtained in 1.5 minutes. Sequence parameters were as follows: TE (GE/SE)=13.6/50 ms, FOV=400×240 mm3, in-plane iPAT factor=3, matrix size=120×72×8, resolution=3.3×3.3×5 mm3, TI for SPAIR (Spectral Attenuated Inversion Recovery) fat suppression=90 ms, FOVnav = 200 mm, resnav = 64, Flip anglenav = 15º, TR=1.5s. Before motion analysis, the navigator signals during imaging acquisitions were Fourier transformed and truncated to exclude RF saturation along IS direction from the GE/SE readout (Fig. 1). The positon for this truncation was calculated based on peaks fitted from the averaged training navigator and the peaks from the first 32 interleaved navigators. The diaphragm position was derived by calculating the phase difference of the interleaved navigator signals at each acquisition after Fourier transform and truncation. The unwrapped data from different coils were then combined by using coil clustering2 based on the first 32 interleaved navigators. The motion information was then directly sent back to the sequence and slice positioning was adjusted in real-time. This motion analysis and real-time feedback was performed in ICE (Image calculation environment, Siemens Healthcare, Erlangen, Germany).RESULTS
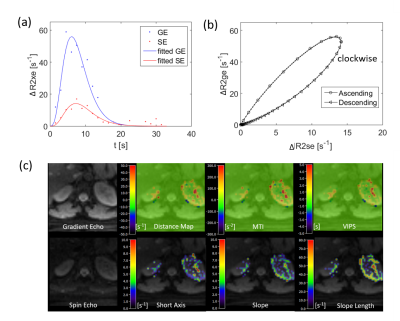
The navigator data showing motion of the abdomen with respiration and the calculated motion information are precisely overlaid on the navigator data (Fig 1a, c). After using real-time motion correction the liver and kidney can be more stably measured in two healthy subjects (Fig 1b, d). The calculated kidney VAI signal curve, hysteresis curve, and parametric maps are shown in Fig 2.CONCLUSION
This study demonstrates the feasibility of navigator-triggered technique in kidney VAI. The respiratory motion from navigator signal can be precisely calculated and slice positioning can be changed in real-time based on the motion information. The sequence may then improve the evaluation of the microvasculature in kidney diseases.Acknowledgements
No acknowledgement found.References
1. Zhang K, Yun SD, Triphan SMF, et al. Vessel architecture imaging using multiband gradient-echo/spin-echo EPI. Plos One. 2019;14(8).
2. Zhang T, Cheng JY, Chen YX, et al. Robust Self-Navigated Body MRI Using Dense Coil Arrays. Magn Reson Med. 2016;76(1):197-205.
Figures