2530
Multiparametric MR imaging in diabetic nephropathy: New insights to evaluate early diabetic nephropathy noninvasively1Radiology, Kawasaki Medical School, Kurashiki, Japan, 2Kawasaki Medical School, Kurashiki, Japan, 3Phillips Japan, Tokyo, Japan
Synopsis
The purpose of this study was to identify the changes in multiparametric magnetic resonance imaging (MRI) findings in early diabetic nephropathy. Measurements were made of the renal cortex and renal medulla T2 values, T2* values and R2* values, as well as optimal TI, inverted TI value and cortico-medullary difference on optimal TI in SSFP with ssIR pulse with multi TI. Also, renal cortical thickness and renal length were measured, representing morphological changes. Significant differences were seen between the healthy and early diabetic nephropathy (grade1 and grade2) in T2 values of cortex and T2* values of medulla. This study suggests the possibility that MRI using the values of T2 of cortex and T2* of medulla can be used to evaluate early diabetic nephropathy non-invasively and in a short period of time.
Introduction
The incidence of diabetic nephropathy is increasing, and it has recently become the most common underlying disorder in dialysis patients. Early stages diabetic nephropathy is difficult to diagnose with regular kidney function tests, and at present, the severity is determined based only on microalbuminuria. More accurate diagnosis requires evaluation with multiple tests. In order to perform these tests in a larger number of early stages diabetic patients who have few symptoms, the tests should ideally include those that can be performed non-invasively and in a short period of time. The purpose of this study was to identify the changes in multiparametric magnetic resonance imaging (MRI) findings in early diabetic nephropathy.Methods
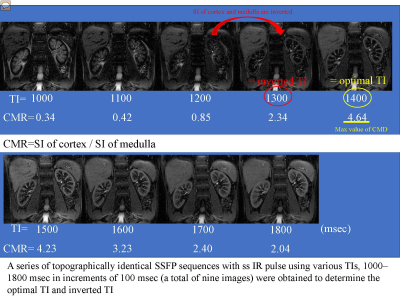
The study subjects (N=51) included 42 patients hospitalized with diabetes between December 2018 and November 2020 who had diabetic nephropathy and who consented to participate in this study (Stage 1: 26 patients, Stage 2: 8 patients, Stage 3: 5 patients, Stage 4: 3 patients), and 9 healthy volunteers. All subjects underwent non-contrast MRI using a 3-Tesla MRI machine. Measurements were made of the renal cortex and renal medulla T2 values, T2* values, R2* values, optimal inversion time (TI) (= TI of maximum corticomedullary contrast ratio (CMR = signal intensity (SI) of cortex / SI of medulla)) and inverted TI value (value of TI that inverts the renal cortex and renal medulla SI) in steady-state free precession (SSFP) with a special selective inversion recovery (ssIR) pulse with multi TI (TI = 1000, 1100, 1200, 1300, 1400, 1500, 1600, 1700, 1800 msec) (Fig. 1), as well as renal cortical thickness and renal length, representing morphological changes. Each of these values was compared among 5 groups; a healthy group (group 0) and 4 stage groups (group 1-4).Results
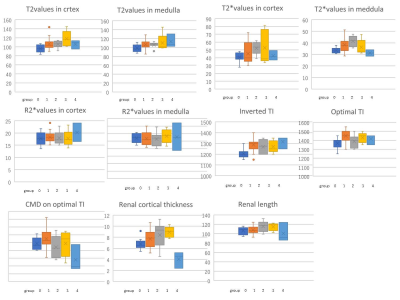
A significant difference between the 5 groups was seen in T2 values of cortex, T2* values of medulla and the value of inverted TI of SSFP with a ssIR pulse with multi TI and renal cortical thickness. In two-group comparisons, significant differences were seen between group 0 and group 1 in values of T2 (mean ± SD: 95.8±8.39 vs. 106.4±11.49; p=0.015) T2* (33.4±2.41 vs. 38.3.0±6.31; p=0.020), inverted TI (1205±49.4 vs. 1288±55.3; p=0.001) and optimal TI (1367±61.2 vs. 1450±60.0; p=0.002). Significant differences were seen between group 0 and group 2 in values of T2 (95.8±8.39 vs. 105.7±6.38; p=0.021) T2* (33.4±2.41 vs. 41.4±4.69; p=0.001) (Fig.2).Discussion
In chronic renal impairment other than diabetic nephropathy, the estimated glomerular filtration rate index used to evaluate kidney function gradually decreases as renal impairment progresses. Morphological changes indicating a gradual decrease in renal cortical thickness and renal length are also seen. In diabetic nephropathy, on the other hand, these indicators are unreliable especially in early stage. Because glomerular hypertension, and conversely are characteristic changes in early diabetic nephropathy. Glomerular hypertension is thought to produce enlargement of the glomeruli and edematous changes in surrounding tissue. In other words, the water content in tissue increases. Values of T2 and T2* showed significant differences between the healthy kidney (group 0) and early diabetic nephropathy (group 1 and 2) were images that can exquisitely was image that can exquisitely capture tissue water content of cortex and hypoxia of medulla as the early changes of diabetic nephropathy. Thus, they might be sensitive indicators of the changes of early diabetic nephropathy. In future, it will be necessary to conduct further investigations with greater numbers of patients.Conclusion
This study suggests the possibility that MRI using the values of T2 in cortex and T2* in medulla, which can sensitively capture edematous changes in the renal cortex and hypoxia in renal medulla, can be used to evaluate early diabetic nephropathy non-invasively and in a short period of time.Acknowledgements
No acknowledgement found.References
1. Wolf M, de Boer A, Sharma K, et al. Magnetic resonance imaging T1- and T2-mapping to assess renal structure and function: a systematic review and statement paper. Nephrol Dial Transplant. 2018 Sep 1;33(suppl_2):ii41-ii50. 2. Noda Y, Ito K, Kanki A, et al. Measurement of renal cortical thickness using noncontrast-enhanced steady-state free precession MRI with spatially selective inversion recovery pulse: Association with renal function. J Magn Reson Imaging. 2015 Jun;41(6):1615-21. 3. Hall ME, Jordan JH, Juncos LA, et al. BOLD magnetic resonance imaging in nephrology. Int J Nephrol Renovasc Dis. 2018 Mar 13;11:103-112. 4. Parving HH, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner BM, ed. Brenner and Rector’s The Kidney. 8th ed. Philadelphia, PA: Saunders Elsevier, 2007; 1265-1298Figures