2512
Inductively coupled small-diameter volume coils insertable to the knee coil at 7T for MR microscopy1Human Brain Research Center, Kyoto University, Kyoto, Japan, 2Quality Electrodynamics, Mayfield Village, OH, United States, 3Department of Human Health Sciences, Kyoto University, Kyoto, Japan, 4Department of Neurology, Kyoto University, Kyoto, Japan
Synopsis
MR imaging is frequently correlated to histopathology of specimen, and their high-resolution imaging is required. However, it is not usually feasible using the whole-body human MR scanner. We implemented inductively coupled small diameter coils insertable to the knee coil at 7T without modification of the coil interface. Up to isotropic 50 μm imaging was successfully conducted, and fine details of the specimen could be visualized using the same sequence for in vivo scans. The proposed coils are easy to use and will facilitate investigation of image-histopathology correlation.
Introduction
The human 7T MR scanner is gradually becoming widely available and enables high-resolution imaging using latest pulse sequences. Validation of image finding on specimen or animal model is occasionally required, and some animal systems are used to scan small samples. However, some pulse sequences are provided solely to a human scanner. If we could visualize them using the same human MR scanner, it is highly convenient. At 7T, 3 times higher SNR than at 3T can be attained, but it requires an appropriate coil.1 In addition, latest human MR systems have a multi-channel capability. Building multi-array coils2-6 are possible options, but construction of such a multi-element coil is not always feasible. However, the latest system is usually furnished with large-sized multi-element coils. If a small-size coil with high local sensitivity can be inserted and used in the human coils without wiring, it would be highly advantageous.A small-size volume coil has been used to scan small objects with a very high SNR. If designed appropriately, a small-size volume coil can be used inductively coupled with a larger-size coil without wiring. Here, we fabricated an unplugged insert-type volume coils and examined their capability to visualize small objects using a human 7T MR system.
Methods
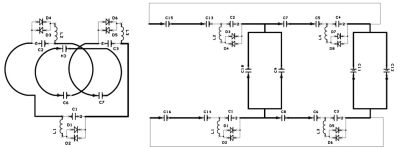
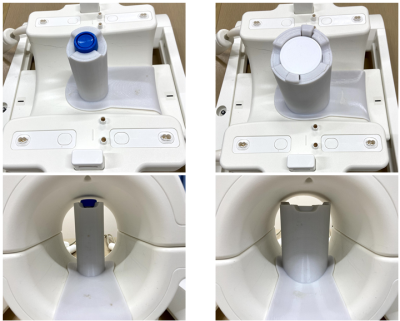
An investigational whole-body human 7T scanner (MAGNETOM 7T, Siemens Healthineers, Erlangen, Germany) and a Tx and 28-ch Rx knee coil (Quality Electrodynamics, Mayfield Village, OH, USA) was selected as the baseline system. The inner diameter of the knee coil is 150mm. Unplugged insert-type volume coils were designed to fit into the knee coil with diameter of 26 and 64 mm (D26 and D64 coils). The D26 coil was consisted with a 3-turn solenoid coil (total length: 500 mm, three passive only decoupling circuits). The D64 coil was consisted with a quadrature saddle coil and capacitive decoupling network (total length: 8 cm, four passive decoupling circuits). See Figure 1. Placement of these coils inside of the knee coil is presented in Figure 2. No wiring was required. During the knee coil transmit, the insertable volume coils were detuned by the built-in passive decoupling circuits and no B1 field enhancement or distortion induced by the insertable volume coils. When the knee coil was in receive state, the insertable coils were inductively coupled to the 28ch knee receive array coil and generate a high sensitivity and undistorted B1 field distribution within the localized resonator.Ex vivo brain specimen were measured soaked in proton-free susceptibility-matching fluid (Fluorinert FC-43, 3M Corp., MN, USA) using a 3D gradient-echo sequence for T2*-weighted images. An excised brain part of an Alzheimer’s disease (AD) patient and a macaque whole brain were scanned using the D26 and D64 coils in isotropic 50 μm resolution (FOV 20 mm, TR/TE 200/23 ms, BW 40 Hz/pixel) and 150 μm resolution (FOV 57 mm, TR/TE 100/20 ms, BW 40 Hz/pixel), respectively. Scan time was approximately 4 hours for each scan.
Results
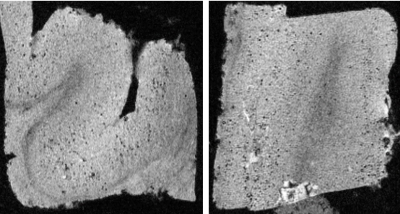
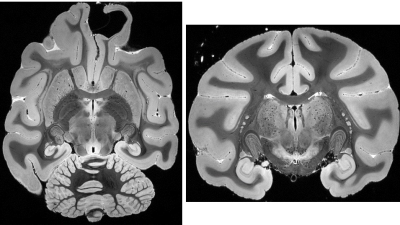
Representative images of the specimen are shown in Figures 3 (D26) and 4 (D64). In Figure 3, many small dark holes are observed located at the cerebral cortex of the AD patient. These are considered to be amyloid plaques (50 – 100 μm) with iron deposition. The small plaques could be visualized on T2*-weighted images because of blooming effect caused by their high susceptibility in addition to high spatial resolution. In Figure 3, fine laminar structures of the hippocampus and lateral geniculate nucleus can be observed. Structural details of the cerebellar folia, brain stem, basal ganglia and thalamus are also depicted.Discussion
High-resolution imaging on a human 7T-MRI system was feasible, when the unplugged insertable volume coils were used. They can be used with the knee coil without modification of the coil interface, and their setup is very easy. Scan parameters need to be optimized dependent on the sample size and target contrast. The recommended scan is T2*-weighted imaging because narrower BW is required to retain signal from very small voxels. The long axis of the coils needs to be placed orthogonal to the B0 direction, which is the direction of the knee coil diameter of approximately 15 cm. Even though the current configuration of insert-type volume coils has a limited flexibility of sample size and orientation, that could be improved with different configuration of inductively coupled resonators such as birdcage coils or dielectric resonators7 which could accommodate horizontal bore access for in-vivo animal scans under anesthesia. The inductively coupled small-diameter volume coils may perform well even at 3T and 1.5T, although it is left to be validated.Conclusion
Inductively coupled small diameter coils that can be inserted to the knee coil could successfully visualize fine structures of the specimen. They are easy to handle and enable MR microscopy using the same whole-body 7T-MRI system that is used for in vivo scans.Acknowledgements
This research was supported by the Agency for Medical Research and Development, Japan (Grant Number: JP20dm030700).References
1. Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016; 75:801-809.
2. Harteveld AA, Denswil NP, Siero JC, et al. Quantitative Intracranial Atherosclerotic Plaque Characterization at 7T MRI: An Ex Vivo Study with Histologic Validation. Am J Neuroradiol. 2016; 37:802-10.
3. Zhang B, Sodickson DK, Cloos MA. A high-impedance detector-array glove for magnetic resonance imaging of the hand. Nat Biomed Eng 2018; 2:570-577.
4. Edlow BL, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data 2019;6:244.
5. Urayama SI, Zhang B, Fujimoto K, et al. A modular 7T high-impedance array for ex-vivo imaging. In Proc Int Soc Magn Reson Med 2019, 1451. Montreal, Canada.
6. Fujimoto K, Zhang B, Urayama S, et al. A tight-fit flexible high-impedance coil array for high-resolution imaging of small ex-vivo specimen using a human 7T scanner. In Proc Int Soc Magn Reson Med 2020, 4018. Paris, France.
7. Webb A. Dielectric materials in magnetic resonance. Concept in Magn Reson Part B 2011;48:148-184.
Figures