2511
Optimized Single-loop Coil with 3D-shaped Design for Simultaneous fMRI and Optical Imaging in Rodent1Biomedical Engineering, Emory University/Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Single loop coil was optimized in 3D shape to fitting rodent bran geometry and designed in slim and large center opening to meet the requirement of simultaneous fMRI and optical imaging. Relative to conventional 2D flat loop, the proposed 3D design may allow setting a loop coil closer to rat cerebral hemispheres and save more overhead space for optical imaging configuration than a 2D flat coil while keeping B1 perpendicular to B0. Our 3D-shape modification exhibited significant improvement in overall SNR and signal homogeneity especially in cortical areas and center brain in T2-weighted images, EPI and field maps.
INTRODUCTION
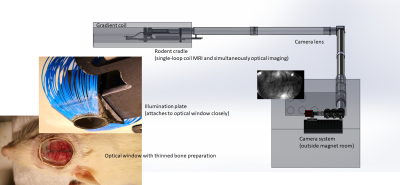
Both fMRI and optical imaging are current powerful neuroimaging tools to investigating brain function. Optical imaging has the great advantages of cellular type-specific indicators and multiple-parameter measurements of hemodynamic signals but is limited to cortical surface coverage1,2. In contrast, fMRI provides noninvasive whole-brain coverage but is limited to hemodynamic signals. The combination of the two different techniques in one experimental setting would be ideal for exploring brain function3,4. In practice, as demonstrated in Fig.1, an optical imaging configuration requires an open/clear area over the rodent head for illumination and detection of optical signals. This can be challenging for MRI coil choice and positioning during simultaneous optical/MRI studies. Current multi-channel-coil-arrays or cryoprobes are not well-suited for simultaneous optical imaging. A single-loop coil with a large open space in the center area and a slim design to reduce spatial constraints is one feasible option, and has been used in prior multimodal studies that combine fMRI and microelectrode recording5. To improve upon this approach by optimizing brain coverage and SNR for transmit/receive single-loop surface coils, we designed a 3D loop coil specifically for the anatomy of the rat head, which surrounds the brain volume as closely as possible, demonstrated a significant improvement in image quality in our preliminary studies.METHODS
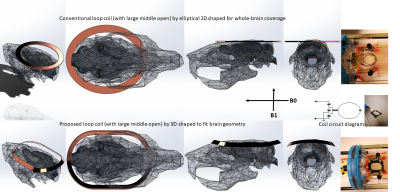
Based on the geometry of the rat head/brain (250-300g/SD/male), an optimized loop shape in 3 dimensions was developed in SolidWorks CAD software, illustrated in Fig.2. Typical surface coils with large middle-openings are mostly circular or ellipse-shaped and flat (2D). We have previously observed that ellipse-shaped coils can have better coverage along the nose-to-tail dimension of the rat brain and better overall signal homogeneity (not shown). Our design added a third dimension of depth to an elliptical 2D-coil, adding a slight saddle shape to bring the left and right sides of the coil closer to the brain. The 3D-coil was based on the design of an existing 2D elliptical coil (24mmX30mm with conductive strip width of 3mm on PCB). The 3D-coil shape were bent along the short side wing of 24mmX30mm elliptical loop into 76mm-diameter circular volume, and the side wings were additionally reduced to straight lines (20mm side-to-side width). In addition, the coil strips are formed in 45-degree positioning for roughly parallel facing to the brain from all directions. The coil base was printed out in epoxy resin and copper tape strip was attached to the formed edge and soldered to a 2pF capacitor at very end and connected to cable at another end (finished with a layer of epoxy for insulation). The connected cable was about 30mm long, twisted and shielded to ground, before connecting to match/tuning trimmers (1-12pF variable capacitors). Both 2D- and 3D-shaped coils were tested in rats (20 rats with the 2D-coil, 3 rats with the new coil, and 1 rat with both sequentially) in a Bruker 9.4T scanner with identical scan settings/parameters, under 2% isoflurane anesthesia. Single-shot gradient echo EPI: TR=1200ms/TE=15ms for 24 axial continuous slices, voxel size=isotropic-400um. The surrounding muscle/skin signals were saturated using four sat-slices. T2-weighted: RARE, TR/TE=3500ms/11ms, RareFactor=10, with same geometry as EPI scan.RESULTS
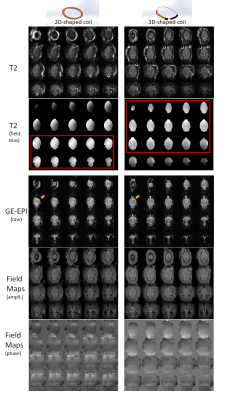
The T2-weighted images, EPI and field maps of the same animal are shown in Fig.3 for comparison. Although both coils cover most of the brain, the 3D-shaped coil data shows better overall field homogeneity and SNR. From the field-bias map of T2, calculated by N4ITK6, the 2D-flat-elliptical coil exhibits good coverage and field homogeneity in the lower brain but less field homogeneity in central/upper brain. Similar patterns of homogeneity were observed in the other rats imaged with only the 2D-coil. In contrast, the 3D-shaped coil exhibits excellent field homogeneity in central brain, including top and middle areas that are of primary interest for simultaneous optical and MRI studies, although there is less homogeneity lower brain slices than for the 2D-coil. EPI obtained with the 3D-coil has less signal dropout/distortion compared to the 2D-coil, especially in prefrontal cortex and the cerebellum. We compared SNRs (0.655*tissue signal mean/air signal std7, calculated from the field map amplitudes with high signal homogeneity to avoid bias from ROI selection) and obtained 51.8 for 3D vs. 42.4 for 2D in cortex; 50.6 for 3D vs.45.0 for 2D in subcortical areas; and 43.6 for 3D vs. 43.3 for 2D in cerebellum. The differences in field map phases can be identified by visual inspection, and exhibited better homogeneity in the main brain areas for the 3D-coil.DISCUSSION/CONCLUSION
The single loop transmit/receive coil has unique advantages of volume and positioning flexibility, as it takes little space and has a large middle open area well-suited for simultaneous optical imaging and MRI. We demonstrated a 3D-formed loop coil with assistance of CAD design/3D-printing based on rodent skull/brain anatomy for optimized positioning near the brain. The major improvement from the 3D-shaped coil allows the coil to set closer to the cerebral hemispheres while keeping B1 perpendicular to B0. The field-bias maps indicate the 3D-shaped coil may "focus" effectively on the central brain area for nearly uniform coverage. The comparisons indicate the 3D-coil has overall better SNR in most brain and improved signal homogeneity, particularly in the cortical areas that will be observed with optical imaging.Acknowledgements
Grant sources (NIH): 1R01MH111416, 1R01NS079095, 1R01EB029857References
1. Ma, Y. et al. Wide-field optical mapping of neural activity and brain haemodynamics: Considerations and novel approaches. Philosophical Transactions of the Royal Society B: Biological Sciences vol. 371 (2016).
2. Knöpfel, T. & Song, C. Optical voltage imaging in neurons: moving from technology development to practical tool. Nature Reviews Neuroscience vol. 20 719–727 (2019).
3. Howarth, C., Mishra, A. & Hall, C. N. More than just summed neuronal activity: how multiple cell types shape the BOLD response. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 376, 20190630 (2021).
4. Lake, E. M. R. et al. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat. Methods 17, 1262–1271 (2020).
5. Pan, W. ju et al. Simultaneous fMRI and electrophysiology in the rodent brain. J. Vis. Exp. 1901 (2010) doi:10.3791/1901.
6. Tustison, N. J. et al. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
7. Henkelman, R. M. Measurement of signal intensities in the presence of noise in MR images. Medical Physics vol. 12 232–233 (1985).
Figures