2510
Increased B1+ efficiency of a dipole antenna compared to a loop coil for 31P-MRS at 7T: simulations and cardiac MRSI data1Wolfson Brain Imaging Center, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 3Tesla Dynamic Coils, Zaltbommel, Netherlands, 4Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom
Synopsis
We evaluated the performance of two RF coil configurations for ultra-high field (7T) 31P-MRS applications in the body. We tested: (A) a dipole Tx/Rx + loop Rx coil vs (B) a quadrature dual-loop Tx/Rx coil. We assessed their relative performance by EM modelling simulations and through phantom and in vivo cardiac scans. Both simulations and experimental verifications indicate that there is notable improvement in terms of B1+ efficiency for the dipole+loop combination compared to quadrature loops. We extrapolate these preliminary findings to predict the performance of the dipole+loop combination if driven at 35kW total like our previous whole-body birdcage coil.
Introduction
Ultra-high field leads to a 2.5-fold increase in SNR [1] and increased spectral resolution [2]. The use of dedicated receive-arrays further improves the SNR and extends coverage to more of the heart at 7T. Array coils for cardiac 31P-MRS have mostly been using surface coils for transmission. Surface coils’ radiofrequency (RF) transmit field strength (B1+) drops-off rapidly with increasing distance from the coil to the volume of interest [3] which makes liver and heart applications challenging. To overcome one of the main technical challenges of significant B1+ signal dropouts at UHF, different RF coil designs have been introduced, that counteract B1 field variations.In this study we have compared the performance of a dipole Tx/Rx + loop Rx RF coil with a quadrature dual-loop Tx/Rx RF coil configurations for ultra-high field (7T) 31P-MRS applications in the body. We assessed their relative performance through simulations and through phantom field map acquisitions and in vivo cardiac scans.
Methods
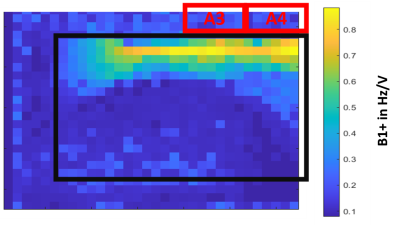
Materials:The surface quadrature Tx/Rx 1H/31P loop coil consists of a1H loop (Frequency = 297.8 MHz) in the centre of the coil and two 31P loops (Frequency = 1210.3 MHz) located on the sides (Figure 1). The new prototype surface body coil dipole array contains 4 anterior and 4 posterior Tx/Rx 1H/31P dipoles. For in vivo data acquisition, dipoles A3 and A4, as shown in Figure 1, have been used for transmission.
Simulations:
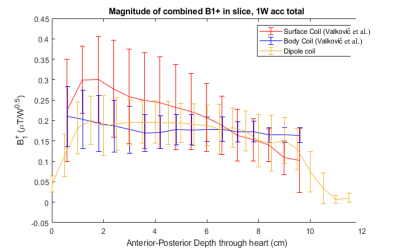
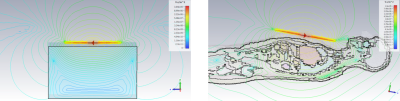
We have performed EM simulations using CST Studio Suite 2020 to compare the transmit efficiency of the dipole design against a whole-body birdcage transmit design (previously reported in Valkovic et al, PlosONE) in terms of B1+ and depth. The coil B1+ efficiency was simulated over a homogenous numerical phantom and a human model with values extracted for all “heart” voxels afterwards (Figure 2).
B1 map acquisition:
A 31P B1 map was acquired from the surface body dipole array coil with a progressive saturation method on a 30 L phosphorus phantom of phosphate concentration of 30 mM. A 3D gradient echo sequence has been used with TR = 100 ms, TE = 5 ms, rectangular excitation pulse of 10 ms length duration, 200 averages and various excitation pulse voltages varying from 2 V to 51.9 V.
MRSI acquisitions:
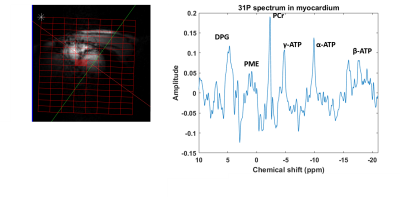
3D weighted CSI cardiac data has been acquired on the heart of a healthy volunteer with TR 1s, matrix size 8x16x8, FOV 220 mm, vector size 1024 points, spectral bandwidth 6 kHz and both dual tuned 1H/31P quadrature loop surface transmit coil and dipole array body coil. Acquisition times were 11 min 48 s.
Results
Figure 2 shows simulations of a B1+ evolution with depth for the novel prototype dipole coil design and for a previous whole-body birdcage coil design (Valkovic et al., PlosONE).Figure 3 shows simulations of B1+ field distribution over a numerical phantom and numerical model of human heart.
Figure 4 shows the acquired B1+ map of the dipole array coil. At typical depths for the heart (5-10cm), B1+ values are about 0.1-0.2 Hz/V. At max power, which for this coil is 600 VRMS (approx. 8kW), we would have a B1+ of about 3.5-7 uT. From Figure 2, at typical depths for the heart, we have a B1+ of about 0.2-0.15 uT/sqrt(W). At maximum power, this corresponds to a B1+ of about 3.7 – 4.9 uT with an 8kW RF amplifier.
Figure 5 shows a spectrum acquired on the heart of healthy man from a 3D CSI acquisition. All the expected cardiac metabolites are clearly visible in this spectrum. The PCr/ATP ratio is as expected for a healthy subject allowing for partial saturation and the usual levels of blood contamination.
Discussion
B1+ field map acquisitions agree with EM modelling simulations for dipole coils. Simulations show that with a set of 4x dipoles, good B1+ homogeneity is obtained right across the chest, similar to our previous whole-body birdcage coil design [1]. Figure 3 shows that with a suitably upgraded RF power amplifier, this coil would have an excellent liver and heart performances comparable to whole-body birdcage designs but without the complexities of mechanical integration into the existing bore liner or mechanical clashes with the standard patient table. The B1+ field values measured in the phantom agree with EM modelling simulations, as shown in Figure 2. These first simulations and experiments make us confident to proceed with planned liver and cardiac studies using this coil. Preliminary human data are shown in Figure 5.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 801075. We would also like to acknowledge Tesla Dynamic Coils for their work in constructing the surface dipole array coil as part of the NICI consortium.
Christiopher T. Rodgers and Ladislav Valkovic are funded by the Wellcome Trust and Royal Society [098436/Z/12/B].
We acknowledge the NIHR Cambridge Biomedical Research Centre and the MRC Clinical Research Infrastructure Award for 7T research.
References
[1] Rodgers, Christopher T., et al. "Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla." Magnetic resonance in medicine 72.2 (2014): 304-315.
[2] Purvis, Lucian AB, et al. "Phosphodiester content measured in human liver by in vivo 31P MR spectroscopy at 7 tesla." Magnetic resonance in medicine 78.6 (2017): 2095-2105.
[3] Rodgers, Christopher T., and Matthew D. Robson. "Receive array magnetic resonance spectroscopy: whitened singular value decomposition (WSVD) gives optimal Bayesian solution." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 63.4 (2010): 881-891.
[4] Valkovič, Ladislav, et al. "Using a whole-body 31P birdcage transmit coil and 16-element receive array for human cardiac metabolic imaging at 7T." PLoS One 12.10 (2017): e0187153.
Figures