2505
Flexible 32-Channel 13C MRI Head Array: An EEG-Lookalike Design Approach1Department of Health Technology, Technical University of Denmark (DTU), Kgs. Lyngby, Denmark, 2Department of Electrical Engineering, Technical University of Denmark (DTU), Kgs. Lyngby, Denmark
Synopsis
We propose a design concept for flexible human head coil arrays applicable to a wide frequency range. Electrically, the design relies on high decoupling obtained through a controlled high mismatched connection to the low-impedance preamplifiers. Mechanically, the array is built into a neoprene EEG cap, and made of regular-copper flexible wire. The array layout is designed to have an axis to stretch along, therefore allowing tight fit to a variety of human-head sizes. A 32-channel prototype for 13C at 3T (32.1 MHz) is presented and evaluated, showing SNR performance superior to a 13C-dedicated volume coil.
Introduction
Hyperpolarized 13C metabolic imaging is emerging as an important tool to investigate the human brain, both in the healthy and diseased1,2. Even with the large increase in available polarization provided by dissolution DNP3, the SNR available in 13C brain metabolic imaging is still limited, and efforts on maximizing the sensitivity of receiver arrays are still necessary. The low frequency of 13C compared to 1H, makes receive arrays of very high number of channels not necessarily beneficial when compared to volume coils, due to high electronic noise and poor sample loading4. In this work, we propose a design of a 13C human head receive array, where we maximize the sample loading by making the whole array flexible, and integrating the array into a neoprene headcap (like those used for EEG). The individual coil elements are made of standard copper wires, and high mismatch to the preamplifiers is created to generate high decoupling, as described in5. The coil elements are sewn into the neoprene headcap in such a way that the headcap can be stretched along the sagittal axis of the array, therefore allowing tight fit to different head sizes. The SNR provided by the array is measured over the whole brain region of a human head phantom, and the results compared to a state-of-the-art volume coil show considerable SNR improvement across most of the region of interest.Materials and Methods
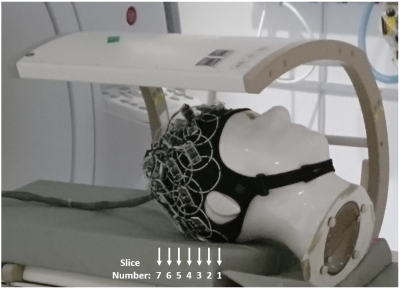
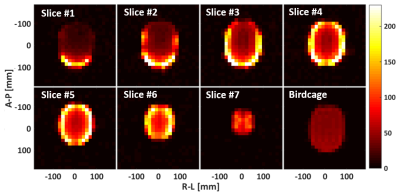
The individual elements forming the array are made of non-segmented loops, with all electronics integrated into a single PCB5. 1H traps are also added to improve compatibility with external 1H coils (needed for anatomical mapping to support 13C metabolic imaging). A coil element together with the PCB is shown in Fig. 1. 32 coil elements are sewn into an EEG neoprene headcap (Artinis Medical Systems). This headcap adapts to heads of 53 to 61 [cm] perimeter, equivalent to sizes XS to L of standard helmet sizing. Two symmetric assemblies of 13 elements (coil diameter of ≈ 65 [mm], wire thickness of 1.7 [mm]) are placed on the left and right sides of the array. Between these two assemblies, there are 6 elements made of a thinner wire (thickness = 0.99 [mm]). All coils are overlapped to their nearest neighbors. With this combination of coil elements of different wire thickness, a preferred axis for stretching is given to the array, which, in this case, is the sagittal axis (dividing the array into left-right). A typical unloaded-to-loaded Q-ratio for the individual elements was measured to be QU/QL=220/80 when loaded with a human head. 13C MRS measurements (CSI, 360×360×20 mm3, matrix size = 24×24) were performed on a Specific Anthropomorphic Phantom (SAM) to evaluate SNR performance4. The measurements were repeated over 7 axial slices separated by 20 [mm], covering the whole brain area of the phantom, as shown in Fig. 2. The performance of the array was compared with a volume coil of the birdcage type (RAPID Biomedical).Results and Discussion
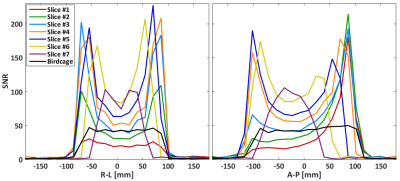
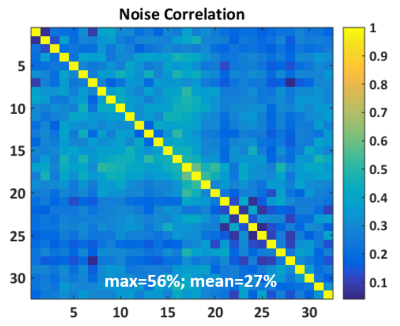
The measured SNR maps are shown in Fig. 3. The coil array shows uniform performance across the whole brain region, with a superficial SNR of around 200 compared with 45 for the birdcage. Fig. 4 shows the SNR profiles across the central axes (right-left, anterior-posterior). The central SNR varies for the different slices, which is expected since the distances from the phantom center to coil elements differ among different slices. Overall, the SNR provided by the array is superior in most of the brain volume with only slices 1 and 2 presenting areas with lower performance than the reference volume coil. There is some variation within the noise correlation matrix of the coil array (Fig. 5), and slightly higher values are observed for coils 14 to 19. This is expected since these are the six elements of the array central axis, which are slightly bigger than the rest. Overall, the 27% mean of correlation for the whole matrix is reasonable for a flexible array.Conclusion
A flexible 32-channel head array has been developed for 13C at 3 T, which shows superior performance for human brain imaging when compared to a volume coil also dedicated to human brain. The proposed array can accommodate a variety of head sizes, ranging from size XS to L (53 to 61 [cm] of head perimeter). This result shows that flexible head coils with a high number of channels can be beneficial even for nuclei with low Larmor frequencies like 13C.Acknowledgements
No acknowledgement found.References
1. Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res. 2018. doi:10.1158/0008-5472.CAN-18-0221.
2. Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1–13C]pyruvate and magnetic resonance imaging. Neuroimage. 2019;189:171-179. doi:10.1016/J.NEUROIMAGE.2019.01.027.
3. Ardenkjær-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158-10163. doi:10.1073/pnas.1733835100.
4. Sánchez-Heredia JD, Olin RB, McLean MA, et al. Multi-site benchmarking of clinical 13C RF coils at 3T. J Magn Reson. 2020;318:106798. doi:10.1016/j.jmr.2020.106798.
5. Sánchez-Heredia JD, Johansen DH, Hansen RB, et al. Improved Decoupling for Low Frequency MRI Arrays Using Non-Conventional Preamplifier Impedance. IEEE Trans Biomed Eng. 2019;66(7):1940-1948. doi:10.1109/TBME.2018.2881203.
Figures