2504
A nested dual-tuned proton-sodium loop-array transceiver coil on a 9.4T whole-body MRI system1State Key Laboratory of Brain and Cognitive Science, Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 3Institute of Electrical Engineering Chinese Academy of Sciences, Beijing, China, 4University of Chinese Academy of Sciences, Beijing, China, 5CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Beijing, China, 6Center for MR Research and Departments of Radiology, Neurosurgery and Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 7Department of Biomedical Engineering, State University of New York at Buffalo, Buffalo, NY, United States, 8Beijing Institute of Brain Disorders, Beijing, China
Synopsis
In this study, a nested dual-tuned proton-sodium multi-channel loop-array transceiver coil was designed and constructed for 9.4T MRI, which could provide images for both proton and sodium at the same location. This coil adopts nested structure, which contains 8-channel transceiver loop elements respectively, and each loop is equipped with an independent transmit/receive circuit. The coil array was simulated for B1 field distribution and was further tested on a 9.4T whole-body MRI platform with home-built spectrometer. Proton and sodium images on a water phantom were successfully collected on this system with high quality.
Introduction
Sodium magnetic resonance imaging (MRI) has been used to detect metabolic properties and diagnose diseases in various living organs [1]. However, the intrinsic concentration of sodium in human body and its MR signal sensitivity are much lower than proton, therefore it is difficult to obtain meaningful metabolic images in low-field MRI. Ultrahigh field MRI has intrinsic high signal-to-noise ratio (SNR), therefore can offer substantial benefits to obtain high-quality image of hetero-nuclei in metabolic study [2-3]. To co-register the sodium and proton images in a single setting, a nested dual-tuned loop-array radiofrequency (RF) coil was proposed without repositioning the subject, which can sufficiently save scanning time and provide convenience for patients [4-6].Methods
The proton-sodium double-resonant transceiver array coil was specially designed for a 9.4T whole-body MRI as shown in Fig.1. This coil adopts with double-layer cylindrical structure. The outside proton coil array has an inner diameter of 21 cm and 28 cm in length, while the inside sodium coil array consists an inner diameter of 14.5 cm and 23cm in length, each coil array with 8 rectangular loops. The rectangular loop element is 16 cm in length and 8 cm in width with the copper width of 5 mm. Two capacitors are used to eliminate coupling between adjacent loops. The position of sodium and proton loops are shifted by half of the coil width to achieve better inter-frequency decoupling effect. The phantom with an outer diameter of 9.5 cm and a length of 15 cm was placed in the center of the coil, containing 1.25g NiSO4×6H20.5gNaCl per 1 kg of water. The proton and sodium arrays are tuned to the frequency of 400MHz and 106MHz separately and all the loops are matched to 50 ohms.The dual-tuned coil array is connected to a home-built multi-channel RF interface containing a 1-to-8 Wilkinson RF power splitter, 8 fast transmit/receive (T/R) switch and low-noise preamplifier, etc. During transmission, the λ/4 coaxial cable can generate high isolation between the transmitting and receiving pathways to protect the preamplifier. The divided RF signals are shifted linearly by eight coaxial phase shifters, resulting in a 45˚ phase difference among the coil loops to achieve homogeneous B1-field through RF shimming [7-8]. The first-stage amplification in the receiving pathway has noise figure less than 1 dB which can sufficiently suppress noise during acquisition.
An electromagnetic (EM) simulation tool (HFSS, Ansys Ltd.) based on finite element (FEM) approach was used to simulate the field distribution of the proton-sodium dual-tuned coil array [9]. The input power at each port is set to be 1 Watt. The electromagnetic parameters of the water phantom were: conductivity σ = 0.54 S; relative permittivity εr = 74. The dimensions of the coil and phantom models in the simulation are based on the actual coil and phantom size. Finally, the coil array was tested on a 9.4T whole-body MRI scanner (830 cm in bore diameter) equipped with Tesla magnet and home-made spectrometer.
Results
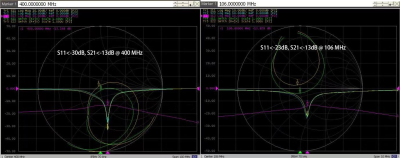
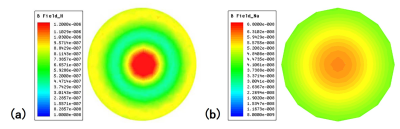
Through laboratory bench tests, all the reflection coefficients S11 of proton and sodium array elements were less than -25dB, and all the transmission coefficients S21 for adjacent elements were less than -13dB with good isolation (as shown in Fig. 2). The isolation between the two arrays was less than -30dB. The laboratory results show that the two coils were tuned to the desired frequencies and decoupled well. The T/R switch had the transmission coefficient of 0.96dB and an isolation degree of -32dB.To assess the field homogeneity of the array coils, the B1 field distribution were simulated and shown in Fig.3 for both proton and sodium. The proton B1 map has a bright spot in the center, with a dark ringing pattern caused by the destructive interference between the coil elements and the water phantom. The sodium B1 field shows a relatively homogeneous distribution in the water phantom. The color scale for proton B1 field covers much larger range than for sodium B1 field.
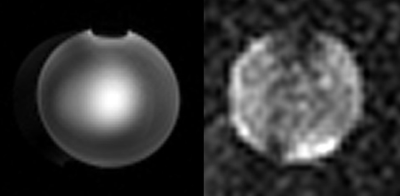
We used fast low-angle shot(FLASH)sequence to evaluate the designed coil array and the resulting proton and sodium images were shown in Fig.4. For proton imaging, the sequence parameters were: TR/TE=500/8ms, matrix size=256x256, planar pixel resolution=1x1mm2; and for sodium imaging, the parameters were: TR/TE=600/4.5ms, matrix size=64x64, planar pixel resolution =4x4mm2. The experimental results exhibited a similar pattern as the simulation results except slight distortion of the center bright spot which may be due to the phase discrepancy during RF shimming. The dark spot at the top edge was generated by the air bubble in the water phantom.
Conclusion
A nested dual-tuned proton-sodium multi-channel loop-array transceiver coil were designed and fabricated at the field strength of 9.4 T. EM simulation was applied to evaluate the B1 field of the coil array. The high-quality proton and sodium image was successfully collected at the 9.4T whole-body MRI scanner by using RF shimming for both proton and sodium nuclei. The setup facilitates co-registration of structural and metabolic images with patient convenience.Acknowledgements
This work was supported by National Major Scientific Equipment R&D Project (Grant No. ZDYZ2010-2).References
[1] Boehmer JP, Metz KR, Mao JT, Briggs RW. Spatial mapping of 23Na NMR signals by two-dimensional rotating frame imaging[J]. Magn Reson Med 1990;16:335-41.
[2] Vaughan T, DelaBarre L, Snyder C, et al. 9.4 T human MRI: preliminary results[J]. Magn Reson Med 2006, 56(6): 1274-82.
[3] Shah N J, Worthoff W A, Langen K J. Imaging of sodium in the brain: a brief review[J]. NMR in Biomedicine, 2016, 29(2): 162-74.
[4] Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 tesla[J]. J Magn Reson 1997;125:178-84.
[5] Kim JH, Moon CH, Park BW, Furlan A, Zhao T, Bae KT. Multichannel transceiver dual-tuned RF coil for proton/sodium MR imaging of knee cartilage at 3 T[J]. Magn Reson Imaging 2012;30:562-71.
[6] Shajan G, Kozlov M, Hoffmann J, et al. A 16‐channel dual‐row transmit array in combination with a 31‐element receive array for human brain imaging at 9.4 T[J]. Magn Reson Med 2014, 71(2): 870-9.
[7] Van de Moortele P F, Akgun C, Adriany G, et al. B1 destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil[J]. Magn Reson Med 2005, 54(6): 1503-18.
[8] Hoffmann J, Shajan G, Scheffler K, et al. Numerical and experimental evaluation of RF shimming in the human brain at 9.4 T using a dual-row transmit array[J]. Magn Reson Mater Phys Biol Med 2014, 27(5): 373-86.
[9] Kozlov M, Turner R. Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation[J]. J Magn Reson 2009, 200(1): 147-52.
Figures