2500
1H, 31P, 23Na, and 13C imaging and spectroscopy with a multi-tunable double-coil assembly1Department of Physics and Engineering, ITMO University, Saint-Petersburg, Russian Federation, 2Faculty of Fundamental Medicine, Lomonosov Moscow State University, Moscow, Russian Federation
Synopsis
The RF-coils of small-animal scanners often limit the range of accessible nuclei to single- or double-nuclear scanning. Here, a multi-tuneable RF-coil design is investigated. The design comprises a butterfly-type RF-coil for 1H, and a multi-tuneable coil for X-nucleus imaging. The coil assembly is tested in a 7 T scanner. 1H and X-nucleus field maps show good matching between the simulation and experiment. Imaging and spectroscopy experiments provide well-resolved images as well as distinctive spectral peaks corresponding to the phantom compounds. The presented imaging method with single coil assembly is considered a promising expansion of heteronuclear imaging.
Introduction
The trend of increasing the static magnetic field in MRI and the associated sensitivity growth has led to the possibility of obtaining in-vivo non-hydrogen nuclei images without isotope enrichment. The transmit/receive systems capable of X-nuclei imaging are commonly present in preclinical small-animal scanners, yet their terminal part (i.e., the radiofrequency (RF) coil) still often limits the range of accessible nuclei by being only single- or double-tuneable. To overcome this limitation and perform heteronuclear imaging with more than two active nuclei (referred here to as multiheteronuclear imaging, in contrast with common heteronuclear imaging, involving only hydrogen and a single X-nucleus) several solutions have been suggested.1–3 Here, an experimental verification of such multiheteronuclear imaging is presented.Methods
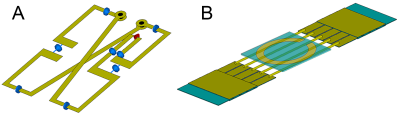
A previously untested multi-tuneable RF-coil design suggested for 11.7 T imaging4 was adapted here for 7 T scanner. The design comprised two separate receive/transmit elements placed on the opposite sides of the animal. The two parts corresponed to the butterfly-type RF-coil for 1H imaging and a multi-tuneable coil for X-nucleus imaging (Fig.1). Adaptation of the 1H-imaging part for lower field strength was performed by modeling the system in CST Microwave Studio and selecting the necessary capacitors to adjust the resonance of the coil to 300 MHz. The redesigned coil was assembled using the same materials as in the original design. The assembled coils were bench-measured using Planar s5048 VNA to confirm the manufactured coil properties conforming to the simulated models.To assess the coil assembly imaging capabilities it was tested in a 7 T Bruker BioSpec 70/30 USR scanner running ParaVision 5.1 and TopSpin 2.0 software. First, the assessment of the coil magnetic field distribution and its comparison with the simulation results was performed. Imaging was done on multi-nuclear block (37×44×154 mm3 internal size) phantom produced according to a 7 T muscle phantom recipe5 with an exception of substituting NaCl with NaH2PO4. The experimental field maps were acquired after tuning the 1H coil part to the 300.3 MHz and the X-coil part to the 79.7 MHz for sodium imaging. In both cases, a series axial and coronal images were acquired using FLASH pulse sequence and 5 to 14 different RF attenuation values.
Next, imaging and spectroscopy capabilities were assessed on two different phantoms: a rectangular DMSO-filled phantom for 13C spectroscopy and a 3-section phantom for 1H and 23Na imaging, and 23Na and 31P spectroscopy. The solutions in 3 sections were variations of the 7 T muscle phantom recipe with NaH2PO4 substituted by MnCl2 and NaCl in different proportions. 1H-imaging was done using a FLASH pulse sequence with TE/TR = 3/150 ms, 1 ms block excitation pulse, 100×100 mm2 coronal plane FoV, 100×100 matrix and a 2 mm-thick slice. 23Na-imaging was done using a FLASH pulse sequence with TE/TR = 3.28/10.45 ms, 0.45 ms block excitation pulse, 100×100 mm2 coronal plane FoV, 128×128 matrix, 8192 averages and a 40 mm-thick slice. 31P-spectra were recorded using a pulse-acquire sequence with a 10 kHz bandwidth, 2048 time points and 64 averages. 23Na-spectroscopy employed a 20 kHz bandwidth, 4096 time points and no averaging. 13C- spectroscopy employed a 15 kHz bandwidth, 4096 time points and 1024 averages. Prior to each measurement the S-parameters of the coil in use were recorded with a Rohde&Schwarz ZVH4 VNA.
Results
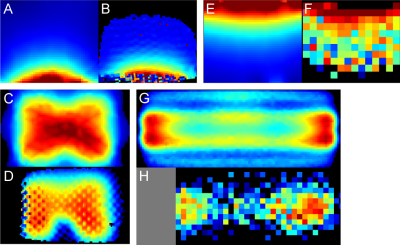
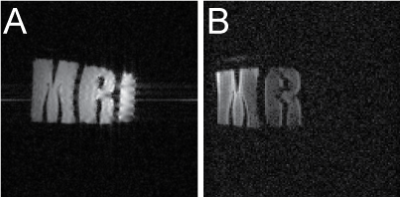
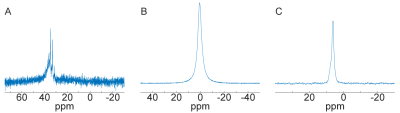
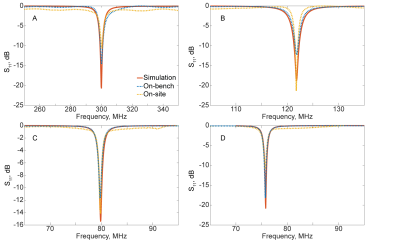
1H and X-nucleus coil field mapping (Fig.2) showed good qualitative matching between the simulated and the measured B1+ fields both in coronal and axial planes, confirming the possibility of using the proposed modified design in lower fields (7 T versus 11.7 T in the original design). Imaging and spectroscopy experiments showed well-resolved images (Fig.3) as well as distinctive peaks corresponding to the expected scanned compounds: single 23Na peak for NaCl and NaH2PO4 solutions, single 31P peak for NaH2PO4 solution and a partially-resolved 13C septet for DMSO (Fig.4).Discussion
The multi-nuclear coil assessment at 7 T has experimentally shown its possibility to be tuned (Fig.5) to a number of frequencies corresponding to a set of nuclei, including 1H, 31P, 23Na and 13C. Field mapping provided a confirmation of the ability of the coil to produce the desired imaging field of view. Imaging data shows the possibility of heteronuclear imaging with the proposed coil design. While further protocol optimization is required to find the optimal conditions for 23Na imaging, a proof of imaging principle with the proposed design has been hereby presented. Spectroscopy data shows the ability of the coil to capture the NMR response from a set of other nuclei (particularly, 31P and 13C). This provides a pathway for further protocol optimization in order to obtain complete MR-images using various X-nuclei, thus allowing for proper multiheteronuclear imaging.Conclusion
The presented proof of principle of multiheteronuclear imaging with single coil assembly opens up a perspective of expanding the experimental capabilities of an MRI scanner. Where previously adding every additional nucleus to the imaging protocol required obtaining an additional RF-coil, providing time for coil interchange, and performing image co-registration, using a single re-tunable coil assembly virtually removes the need for coil interchange and image co-registration. This, in turn, should lead to an increase in the speed, accuracy and the amount of information animal studies can provide.Acknowledgements
This research was supported by the Russian Science Foundation (Project 19-75-10104).References
1. Choi C-H, Hong S-M, Felder J, Shah NJ. The state-of-the-art and emerging design approaches of double-tuned RF coils for X-nuclei, brain MR imaging and spectroscopy: A review. Magn Reson Imaging. 2020;72:103-116.
2. Yang X, Li N, Du F, et al. A four-frequency 1H/19F/23Na/31P RF coil system for simultaneous multinuclear MRI and MRS at 3.0T. In: Proceedings of the 28th Annual Meeting of ISMRM. ; 2020.
3. Kirsch S, Augath M, Seiffge D, Schilling L, Schad LR. In vivo chlorine-35, sodium-23 and proton magnetic resonance imaging of the rat brain. NMR Biomed. 2010;23(6):592-600.
4. Ivanov VA, Hurshkainen AA, Solomakha GA, Zubkov MA. RF-coil with variable resonant frequency for multiheteronuclear ultra-high field MRI. Photonic Nanostruct. 2020;38:100747.
5. Duan Q, Duyn JH, Gudino N, et al. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Med Phys. 2014;41(10):102303.
Figures