2486
MRSaiFE: towards the real-time prediction of tissue heating in MRI - a feasibility study1Department of Radiology, Weill Cornell Medicine, New York, NY, United States, 2GE Healthcare, Aurora, OH, United States, 3Stanford University, Stanford, CA, United States
Synopsis
A crucial safety concern for UHF MRI is the significant RF power deposition in the body in the form of local specific absorption rate (SAR) hotspots, leading to dangerous tissue heating/damage. This work is a proof-of-concept demonstration of an artificial intelligence (AI) based real-time MRI safety prediction software (MRSaiFE) facilitating safe generation of 3T and 7T images by means of accurate local SAR-monitoring at sub-W/kg levels. This feasibility study demonstrates that SAR patterns can be predicted with a root-mean-square error (RMSE) of <11% along with a structural similarity (SSIM) level of >84% for both field strengths.
Introduction
Ultra-high-field (UHF) magnetic resonance imaging (MRI) in clinical and research applications is limited by a key safety concern related to the nonuniform deposition of radiofrequency (RF) power in the body. At UHF, specific absorption rate (SAR) variations can lead to dangerous tissue heating. First, the average SAR is increased since it exhibits a quadratic dependence on the static magnetic field strength (B0). Second, due to the shortened in-tissue wavelength it also exhibits a spatial variation that can lead to dangerous “local SAR” patterns or “hotspots” [2]–[5]. Furthermore, parallel transmit (pTx) technology with multiple independent transmit RF channels [6],[7] commonly used in UHF applications can lead to even stronger hotspots because of potential constructive interference of electric fields. While a small portion of UHF MRI has received first FDA approval for clinical routine (Siemens MAGNETOM Terra [8], GE SIGNA [9]), the vast majority of clinical imaging has been performed at 3T to date. This is largely due to lack of technology that can measure local SAR due to anatomical and positional variations between patients, as well as between transmit coils. Current technology can only determine the overall average/global, SAR, delivered to the entire anatomy under investigation. For this reason, many institutions are required to use a conservative estimate of the peak local SAR via its ratio to the measurable global SAR; typically ~20:1[10]– thereby severely limiting the applied transmit power and thus the imaging performance achievable by UHF MRI. The alternative approach of using MR thermometry suffers from a coarse temperature resolution [11]. In this paper, we propose MRSaiFE, an artificial intelligence (AI) based exam-integrated MRI safety prediction software with the goal of eventually facilitating the safe generation of 7T images. Using this tool, we hypothesize SAR-monitoring at sub-W/kg levels at <10%.Methods
A. Data generation:Input data for this study was acquired from Sim4Life simulations (Zurich MedTech, Zurich, Switzerland) using the Virtual Population (IT’IS Foundation, Zurich, Switzerland).
3T: A 3T body coil model made for a standard bore size of 60cm was used in conjunction with the body models Duke and Ella at 224 different positions spanning from +/-60cm, +/-40cm, and +60/-100cm along the x-, y-, and z- axes (axial: xy-plane, coronal: yz-plane, sagittal: xz-plane). In this first feasibility study, the anatomical input image that would come from an MRI scanner in a real exam was approximated by using a grayscale image of the voxeled body model. The 1g averaged peak local SAR output was evaluated, and coronal SAR slices were extracted (40 slices for Ella, 62 slices for Duke). This resulted in a set of 22,848 input anatomical and 22,848 output SAR images that were used to train the deep learning model, whereof 16,320 were used for training, 4,080 were used for validation, and the remainder for the test dataset.
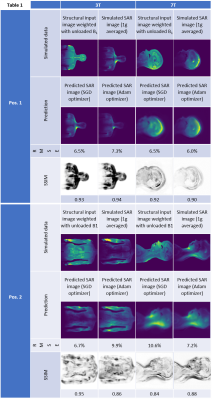
7T: A 7T birdcage head coil model was used with the body model Ella at 175 different positions within the coil ranging from +/-40 cm, +/-20cm, and 60 cm in the x-, y-, and z-direction (axial: xy-plane, coronal: yz-plane, sagittal: xz-plane). Input anatomical image and output SAR image generation followed the same steps as for the 3T analysis using 62 sagittal slices. Example figures of input anatomical image and output SAR image are shown (Table 1).
B. Network:
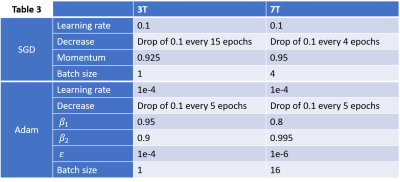
We implemented a unet2D architecture [12] using a cascade of convolutional filters paired with nonlinear reLU activation functions and input image sizes of 224x224 pixels. Training was performed using Adam and stochastic gradient descent (SGD) as well as Keras and Tensorflow (Google, Mountain View, California). Hyperparameters were optimized by minimizing root mean squared error (RMSE) loss on the validation datasets. Training was performed over 30 epochs (SGD) and 6 epochs (Adam) using a GeForce RTX 2080 Ti Graphics Processing Unit (GPU) (NVIDIA, Santa Clara, USA). Layers were randomly initialized using the He initialization.
C. Testing:
Testing was performed on the testing datasets described in “Data generation”. Quantitative image quality comparisons were performed between the ground-truth images (simulated SAR) and the predicted SAR using RMSE and structural similarity (SSIM)[13], which unlike RMSE can evaluate perceptual image quality.
Results
Example predictions are shown in Table 1. Predicted images align well with the ground truth images. A better agreement is seen for the SGD optimizer, though Adam trains faster (Table 2, Table 3).3T results: RMSE values were <10% for all cases with SSIM <=7% in all cases except for Adam in position 2.
7T results: Despite blurriness observed due to alignment issues between input and output data in this first test, RMSE values were found to be <=10% for all cases with SSIM <=7% in all cases except using Adam in position 2.
Discussion and Conclusion
This proof-of-concept study demonstrates feasibility of accurate real-time local SAR-monitoring at sub-W/kg levels at UHF MRI. In practice, MRSaiFE will help to replace the existing conservative SAR margins for optimized, patient-specific values and will free up valuable transmit power that can be used towards better sensitivity, resolution, or scan time. This work could also significantly impact the safety of patients with medical implants [14], in hyperthermia applications [17], and for human MRI at even higher field strengths such 9.4T and 10.5T[15], [16].Acknowledgements
The authors thank Michael Oberle and Erdem Ölfi at Zurich MedTech for their assistance with rapid SAR simulations. This research work was supported by the National Institutes of Health (R00EB024341).References
[1] J. R. Polimeni, B. Fischl, D. N. Greve, and L. L. Wald, “Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1,” NeuroImage, vol. 52, no. 4, pp. 1334–1346, Oct. 2010.
[2] C. M. Collins and M. B. Smith, “Calculations of B1 distribution, SNR, and SAR for a surface coil adjacent to an anatomically-accurate human body model,” Magn. Reson. Med., vol. 45, no. 4, pp. 692–699, Apr. 2001.
[3] M. Thornton, P. Picot, B. Rutt, and S. Winkler, “Method and system for estimating the specific absorption rate of a tissue region prior to a magnetic resonance imaging scan,” US20150316626A1, Nov. 5, 2015.
[4] S. A. Winkler, P. A. Picot, M. M. Thornton, and B. K. Rutt, “Direct SAR mapping by thermoacoustic imaging: A feasibility study,” Magn. Reson. Med., vol. 78, no. 4, pp. 1599–1606, 2017.
[5] S. A. Winkler and B. K. Rutt, “Practical Methods for Improving B1+ Homogeneity in 3 Tesla Breast Imaging,” J. Magn. Reson. Imaging JMRI, vol. 41, no. 4, pp. 992–999, Apr. 2015.
[6] U. Katscher, P. Börnert, C. Leussler, and J. S. van den Brink, “Transmit SENSE,” Magn. Reson. Med., vol. 49, no. 1, pp. 144–150, 2003
[7] M. Pendse, R. Stara, M. M. Khalighi, and B. Rutt, “IMPULSE: A scalable algorithm for design of minimum specific absorption rate parallel transmit RF pulses,” Magn. Reson. Med., vol. 81, no. 4, pp. 2808–2822, 2019.
[8] “FDA Clears MAGNETOM Terra 7T MRI Scanner from Siemens Healthineers.” Siemens Healthineers USA. https://www.siemens-healthineers.com/en-us/news/magnetomterrafdaclearance.html (Accessed: Feb. 10, 2020)
[9] "Bringing Ultra-High Field MR Imaging from Research to Clinical: SIGNA 7.0T FDA Cleared" https://www.ge.com/news/press-releases/bringing-ultra-high-field-mr-imaging-from-research-to-clinical-signa-70t-fda-cleared. (Accessed: Dec. 14, 2020)
[10] “IEC 60601-2-33:2010.” IEC Webstore. https://webstore.iec.ch/publication/2647 (Accessed: Feb. 10, 2020)
[11] V. Rieke and K. B. Pauly, “MR Thermometry,” J. Magn. Reson. Imaging JMRI, vol. 27, no. 2, pp. 376–390, Feb. 2008.
[12] A. S. Chaudhari et al., “Super-resolution musculoskeletal MRI using deep learning,” Magn. Reson. Med., vol. 80, no. 5, pp. 2139–2154, 2018.
[13] Zhou Wang, A. C. Bovik, H. R. Sheikh, and E. P. Simoncelli, “Image quality assessment: from error visibility to structural similarity,” IEEE Trans. Image Process., vol. 13, no. 4, pp. 600–612, Apr. 2004.
[14] M. Etezadi‐Amoli, P. Stang, A. Kerr, J. Pauly, and G. Scott, “Controlling radiofrequency-induced currents in guidewires using parallel transmit,” Magn. Reson. Med., vol. 74, no. 6, pp. 1790–1802, 2015.
[15] D. Shrivastava, T. Hanson, J. Kulesa, L. DelaBarre, P. Iaizzo, and J. T. Vaughan, “Radio frequency heating at 9.4T (400.2 MHz): In vivo thermoregulatory temperature response in swine,” Magn. Reson. Med., vol. 62, no. 4, pp. 888–895, 2009.
[16] J. T. Vaughan et al., “RF technology for human MRI at 10.5T,” in 2013 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), 2013, pp. 1–3.
[17] M. R. Pendse and B. K. Rutt, “Method and apparatus for sar focusing with an array of rf transmitters,” US20160334477A1, Nov. 17, 2016.