2477
Device and simulation workflow for validating cardiac magneto-stimulation thresholds in porcine models1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Cardiovascular Research Center, Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
We developed a magnetic stimulator to measure cardiac stimulation (CS) thresholds of time-varying magnetic fields in pigs. The stimulator consists of a high-power capacitor bank (C=110 µF) discharging into a pancake coil. We will use the measured thresholds to validate a previously developed CS simulation framework that combines electromagnetic field simulations with electrophysiological models of excitable cardiac fibers. We generate whole-body porcine voxel models from multi-bed MRI Dixon and CINE acquisitions to reproduce the anatomy and posture of the animals in our simulations. Carefully validated CS simulations may eventually be useful in setting cardiac safety limits for MRI gradient applications.
Purpose
Time-varying magnetic fields such as MRI gradient fields induce E-fields in the body that can potentially stimulate the heart[1,2]. We have recently developed a simulation framework for the prediction of magnetic cardiac stimulation (CS) thresholds[3-5]. The framework combines E-field simulations in detailed body models with electrophysiological modeling of excitable cardiac fibers. Initial simulations successfully replicated CS thresholds of previous canine experiments performed in 1992[2,6], but a more detailed validation is needed whereby the exact experimental setup (anatomy, posture, coil position, etc.) is correctly represented in our model[3,4]. Here, we report on our ongoing effort to validate our CS simulations in pigs, the primary animal model for the human cardiovascular system[7].Methods
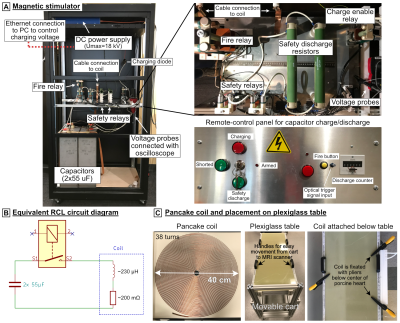
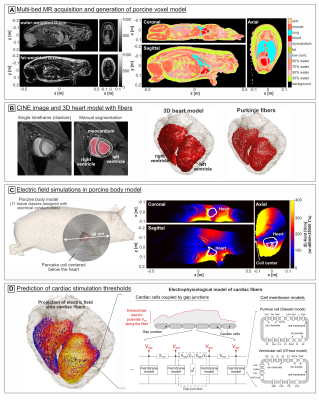
Magnetic stimulator: Figure 1A shows the magnetic stimulator, which is essentially a large capacitor bank discharging into a coil. The capacitors (Umax=18 kV, C=110 µF, HVP, Martinsried, Munich, Germany) are charged by a TDK-Lambda (Tokyo, Japan) FLX-HV power supply. Once the high-power relay (Ross Engineering, Campbell, CA) is closed, the charge disperses into the inductive coil at a resonance frequency of ~1 kHz, and with a decay rate determined by the coil’s AC resistance (~200 mΩ, Fig. 1B). The coil is a flat pancake design (Dout=40 cm, Din=2.5 cm, 38 turns, Fig. 1C) made of 2.3-mm copper wire covered with epoxy and has an inductance of L≈230 µH. Because of the high current/voltages, safety circuits in the pulse generator use additional relays and high-energy resistors for emergency discharge of the capacitors. As an additional precaution, the system is operated by a remote control. The discharge is triggered by the combination of a manual button press (arming the system) and an optical pulse from a laptop monitoring ECG (triggering during diastole). The coil is connected to the pulse generator with a 40 kV-rated 8AWG cable, and a set of GES (Munich, Germany) SB135/HB135 connectors and attached below the table at heart center (Fig. 1C). The amplitude of the discharge pulses will be iteratively increased until the CS threshold is reached, which will be identified and recorded as an ectopic heartbeat on the ECG and SpO2 trace.CS threshold simulations: This study is conducted with approval and under advice of the MGH Institutional Animal Care and Use Committee. The pigs were pre-anesthetized with Telazol and Atropine, intubated, ventilated with O2 (mixed with 1-3% isoflurane), maintained under anesthesia with isoflurane (1-3%), and placed in left lateral recumbency. We acquired fat-water separated Dixon volumes (500x325x344mm3, 2mm isotropic resolution, TR/TE1/TE2=5/1.23/2.46ms) as well as CINE acquisitions (1.3x1.3x2.5mm3, 41 slices, 18 frames, TE/TR=4.9/35.4ms, double oblique along the heart short axis) of the beating heart on a 3T Prisma scanner (Siemens Healthineers, Erlangen, Germany) for seven pigs. Dixon volumes were acquired in 5 bed positions separated by 200 mm and stitched together in postprocessing. We created porcine body models by assigning electrical conductivity values[8] based on the local fat fraction, ranging from pure fat (0.057 S/m) to pure muscle (0.355 S/m). We segmented the lungs (σ=0.11 S/m), assigned the outermost voxel layer as skin (σ=0.17 S/m), and set all voxels with low signal intensity as low-conductive tissue (σ=3.5 mS/m) (Fig. 2A). The heart was manually segmented from the CINE data (diastole frame, Fig. 2B), and cardiac Purkinje and ventricular muscle fibers were added using rule-based modeling algorithms[9,10]. We then simulated E-fields using Sim4Life’s low-frequency solver (Zurich MedTech, Switzerland), and projected the result onto the fibers and integrated to obtain electrical potentials. Finally, we modulated the potential along the fibers over time by the B-field waveform’s temporal rate of change (dB/dt) created by the capacitor discharge and evaluated the electrophysiological fiber models to predict CS threshold values.
Results
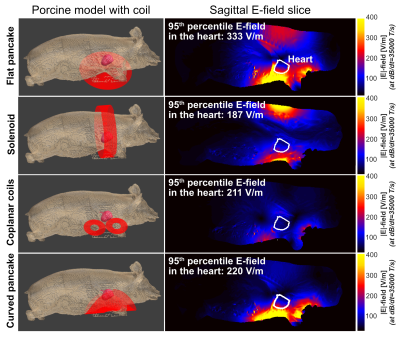
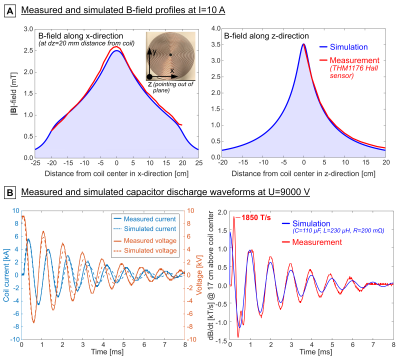
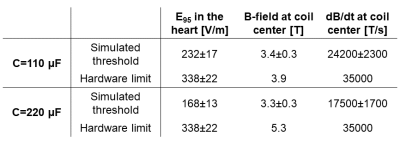
We compared different coil designs for the E-field induced in the porcine heart and found the flat pancake coil design to be the most effective (Fig. 3). Figure 4A shows a comparison between the measured and simulated B-field profiles of this coil. Figure 4B shows the measured and simulated voltage, current, and dB/dt waveforms resulting from the capacitor discharge at U=9 kV (Bpeak≈2.0 T, dB/dtpeak≈17500 T/s at coil center). This corresponds to Bpeak≈3.9 T and dB/dtpeak≈35000 T/s at peak charging voltage (Umax=18 kV).Figure 5 compares CS thresholds simulated in the porcine models with the hardware limits of the stimulator. The average threshold 95th percentile E-field magnitude in the heart of the seven porcine models is E95,thresh=232±17 V/m (compared to E95,peak=338±22 V/m at hardware limit). The average simulation threshold B-field amplitude at coil center is Bthresh=3.4±0.3 T (dB/dtthresh=24200±2300 T/s), which is within the stimulator’s performance limits. Increasing the capacitance to C=220 µF would lower the resonance frequency and decrease the (simulated) thresholds to significantly below the hardware limits (Bthresh=3.3±0.3 T, dB/dtthresh=17500±1700 T/s).
Discussion
CS requires very high magnetic field energies[11], and we previously found that a typical TMS stimulator or MRI gradient would not be powerful enough to guarantee CS[12]. We therefore developed a high-power magnetic stimulator similar to that used in the canine experiments of Mouchawar et al.[2] to validate our CS simulations in pigs. These experiments will ultimately allow us to validate our CS simulations, which could eventually become useful in setting appropriate cardiac safety limits for MRI applications.Acknowledgements
This study is in part supported by the ISMRM Research Exchange Grant and the German Academic Exchange Service (DAAD) as well as NIH award number R01 EB028250.References
[1] Reilly J P, Principles of nerve and heart excitation by time-varying magnetic fields. Ann. N. Y. Acad. Sci., 1992. 649: 96-117.
[2] Mouchawar G A, Bourland J D, Nyenhuis J A, Geddes L A, Foster K S, Jones J T, and Graber G P, Closed-chest cardiac stimulation with a pulsed magnetic field. Med. Biol. Eng. Comput., 1992. 30: 162-168.
[3] Klein V, Davids M, Schad L R, Wald L L, and Guérin B, Investigating cardiac stimulation limits of MRI gradient coils using electromagnetic and electrophysiological simulations in human and canine body models. Magn. Reson. Med., 2021. 85: 1047–1061.
[4] Klein V, Davids M, Schad L R, Wald L L, and Guérin B. Simulation of electromagnetic cardiac stimulation: Validation in dogs and application to human threshold limits for MRI gradient coils. In: Proceedings of the 28th Annual Meeting of the ISMRM. 2020. p. 1125.
[5] Klein V, Davids M, Schad L R, Wald L L, and Guérin B. Simulation of MRI gradient-induced cardiac stimulation: Are the current safety regulations too conservative? In: Proceedings of the 27th Annual Meeting of the ISMRM. 2019. Montréal, Canada; p. 729.
[6] Nyenhuis J A, Bourland J D, Schaefer D J, Foster K S, Schoelein W E, Mouchawar G A, Elabbady T Z, Geddes L A, and Riehl M E. Measurement of cardiac stimulation thresholds for pulsed z-gradient fields in a 1.5-T magnet. In: Proceedings of the 11th Annual Meeting of the SMRM. 1992. Berlin, Germany; p. 586.
[7] Dixon J A and Spinale F G, Large animal models of heart failure. Circ., 2009. 2(3): 262-271.
[8] Hasgall P A, Di Gennaro F, Baumgartner C, Gosselin M C, Payne D, Klingenböck A, and Kuster N, IT’IS Database for thermal and electromagnetic parameters of biological tissues Version 4.0. 2018.
[9] Ijiri T, Ashihara T, Yamaguchi T, Takayama K, Igarashi T, Shimada T, Namba T, Haraguchi R, and Nakazawa K, A procedural method for modeling the Purkinje fibers of the heart. J. Physiol. Sci., 2008. 58(7): 481-486.
[10] Bayer J D, Blake R C, Plank G, and Trayanova N A, A novel rule-based algorithm for assigning myocardial fiber orientation to computational heart models. Ann. Biomed. Eng., 2012. 40(10): 2243-2254.
[11] Schaefer D J, Bourland J D, and Nyenhuis J A, Review of patient safety in time-varying gradient fields. J. Magn. Reson. Imaging, 2000. 12: 20-29.
[12] Klein V, Davids M, Nguyen C, Schad L R, Wald L L, and Guérin B. Feasibility of using transcranial magnetic stimulation devices to study magnetically induced cardiac stimulation in pigs. In: Proceedings of the 28th Annual Meeting of the ISMRM. 2020. p. 111.
Figures