2462
Adequate mixing time for double diffusion encoding in normal brain structures and brain tumors1Radiology, Kyoto Prefectural University of Medicine, Kyoto, Japan, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Healthcare K.K., Shinagawa, Japan
Synopsis
The mixing time for double diffusion encoding (DDE) should be set low to reduce the relaxation effects but also high enough for estimating microscopic fractional anisotropy. We tested the adequacy of the mixing time of 30 msec by comparing acquisitions with parallel and anti-parallel directions as well as with orthogonal and collinear directions. Relatively short mixing time for our cohort was adequate to evaluate the microscopic fractional anisotropy not only in the normal brain area of the white matter and the central gray matter, but also in pathologically abnormal areas such as brain tumors.
INTRODUCTION
Microscopic diffusion anisotropy (μFA) measurements from double diffusion encoding (DDE) promise greater specificity to changes in tissue microstructure compared with conventional diffusion tensor imaging.1, 2 The relative signal intensities (rSIs) of DDE images change depending on the angle between two encoding directions and mixing time. The mixing time is the interval between the two motion-probing gradients and it needs to be set long enough for μFA estimation. However, longer mixing time prolongs the echo time (TE) and results in reduced signal-to-noise ratio. In the past studies, a variety of mixing times were used from 15 msec to 45 msec.3-7 The aim of the study was to test the assumption that a relatively short mixing time of 30 msec is adequate for DDE study on clinical scanner to evaluate the microstructure not only in normal brain structures but also in pathologically abnormal areas such as brain tumors.METHODS
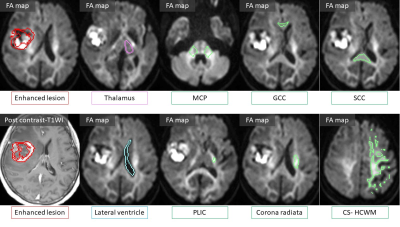
The study was approved by the review committee of our institution. Sixteen patients with enhancing tumors (11 Glioblastomas, 3 Metastases, 2 primary central nerve system lymphomas) were enrolled in the study. Data was acquired on a clinical 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). For clinical purpose, contrast-enhanced 3-dimensional magnetization-prepared rapid acquisition gradient-echo and turbo spin-echo T2-weighted images (T2WI) were obtained in all patients. Additionally, angular DDE measurements were performed with the prototype DDE single-shot echo-planar sequence. In addition to a b0 image, 72 DDE combinations of b2000 images were acquired. The first encodings of the DDE were the twelve directions toward the vertices of the icosahedron. Second encodings were set to the same 12 directions (parallel), 12 opposite directions (anti-parallel), and 48 orthogonal directions (orthogonal) as the first encodings. The mixing time was 30 msec. TR/TE = 6200/146 msec, FOV = 210 mm, pixel size = 1.5 x 1.5 mm2, PAT factor = 2, SMS factor=2, slice thickness = 3mm and acquisition time = 8 min 32 sec or 11 min 1 sec which were related to the slice numbers of 26 or 34, to cover the entire areas with signal abnormality on T2WI.Averaged rSIs of each pixel on the parallel, anti-parallel and orthogonal directions were extracted at the area of enhancing tumor core, normal brain structures of the thalamus, white matter regions such as the genu (GCC) and splenium (SCC) of corpus callosum, the posterior limbs of internal capsule (PLIC), the corona radiata, the centrum semiovale (CS) including high convexity white matter (HCWM) superior to CS, the middle cerebellar peduncles (MCP), and lateral ventricle (Fig.1). The regions of interest (ROIs) for the white matter regions were created by combining all ROIs of white matter structures described above. We compared the rSIs of the parallel and anti-parallel directions and the collinear and orthogonal directions. Data were then analyzed by simple regression analysis and Wilcoxon signed-rank test with Bonferroni correction. We considered P values < .05 to indicate a significant difference.
RESULTS
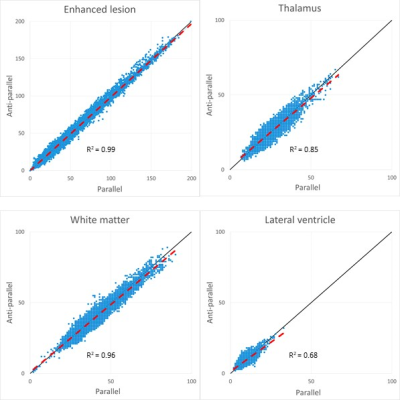
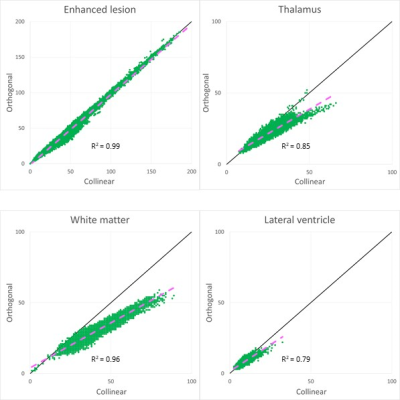
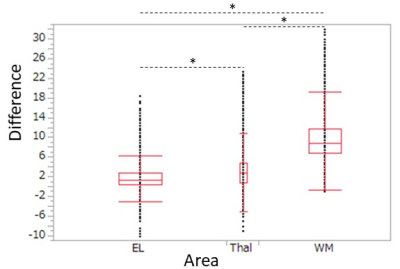
The rSIs of the parallel and anti-parallel directions had highly significant correlations in all areas (p < 0.001) and were close to the straight lines with slope 1, which indicated that they could be equivalent (Fig.2).The scatter plots showed that rSIs of enhanced lesions were found in a wider range than those of normal brain structures and had highly significant correlations (Fig. 2, 3). Although the high correlations were found in the thalamus and the white matter, the rSIs of the orthogonal direction in normal brain structures were lower than those of collinear directions (average of the parallel and the anti-parallel directions) (Fig. 3), and they were significantly lower than those of the enhanced lesions (Fig. 4). The rSIs of the CSF tended to be low and highly correlated in scatter plots.
DISCUSSION
In tissues with high μFA, the signal change corresponding to the DDE angle shows a cosine dependence for short mixing times, and rSIs will increase in the following order parallel, orthogonal, and anti-parallel directions.8 On the other hand, it is known that a long enough mixing time can yield a signal dependence with a W shape, and rSIs of the parallel and the anti-parallel direction are equal, and both of them are higher than those of the orthogonal direction.8 In this study, rSIs of the parallel and the anti-parallel directions were equivalent in all areas, suggesting that the mixing time of 30 msec should be sufficiently long not only in the normal brain tissues but also brain tumors.The results that rSIs of the collinear directions were higher than those of the orthogonal direction may indicate microstructural anisotropy in the normal brain structures, and the absence of this trend in the brain tumors suggested that microstructural changes should have been occurring. We expect to increase the number of clinical cases in the future, which would enable unveiling differences in types and prognosis of brain tumors by using DDE.
CONCLUSION
A relatively short mixing time for DDE is likely to be adequate not only in the areas of normal white matters and central gray matters, but also in pathologically abnormal areas such as brain tumors.Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number JP19K08233.
The authors wish to thank Hiroyasu Ikeno, RT, and Toshiaki Nakagawa, RT, for helping with this study.
References
1. Shemesh N, Özarslan E, Komlosh ME, et al. From single‐pulsed field gradient to double‐pulsed field gradient MR: gleaning new microstructural information and developing new forms of contrast in MRI. NMR Biomed 2010;23:757–780.
2. Callaghan PT. Translational dynamics and magnetic resonance: principles of pulsed gradient spin echo NMR. Oxford,UK: Oxford University Press; 2011. 547 p.
3. Jespersen SN, Lundell H, Sønderby CK, et al. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed. 2013;26(12):1647-62.
4. Lawrenz M, Brassen S, Finsterbusch J. Microscopic diffusion anisotropy in the human brain: Age-related changes. Neuroimage. 2016 1;141:313-325
5. Lawrenz M, Finsterbusch J. Detection of microscopic diffusion anisotropy in human cortical gray matter in vivo with double diffusion encoding. Magn Reson Med. 2019;81(2):1296-1306.
6. Yang G, Tian Q, Leuze C, et al. Double diffusion encoding MRI for the clinic. Magn Reson Med. 2018;80(2):507-520.
7. Kamiya K, Kamagata K, Ogaki K, et al. Brain White-Matter Degeneration Due to Aging and Parkinson Disease as Revealed by Double Diffusion Encoding. Front Neurosci. 2020;15;14:584510
8. Finsterbusch J. Multiple-Wave-Vector Diffusion-Weighted NMR. Annu Rep Spectrosc. 2011;72:225-299
Figures