2455
Evaluation of quantitative MRI parameters reproducibility across a major scanner upgrade: spinal cord diffusion weighted (DW) imaging1NMR research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, London, United Kingdom, 2Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 3Philips Healthcare, Guildford, Surrey, United Kingdom, 4Centre for Medical Image Computing, Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 5E-Heath Centre, Universitat Oberta de Catalunya, Barcelona, Spain, 6Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 7Department of Brain Connectivity Centre Research Department, IRCCS Mondino Foundation, Pavia, Italy
Synopsis
Major MRI scanner upgrades can potentially introduce systematic changes in quantitative MRI metrics and affect reliability and reproducibility of quantitative parameters. Advanced diffusion weighted (DW) imaging methods are a powerful diagnostic and research tool in the brain and in spinal cord, but it is unclear to what extent DW-derived metrics can be affected by upgrades. Here we assess the effect of scanner upgrades on state-of-the-art spinal cord DW techniques. We found that diffusion matrices such as FA, MD and MK remain stable following a major upgrade when measured in the whole spinal cord, white matter, and grey matter areas.
Introduction
Diffusion-Weighted (DW) imaging can be used to characterise tissue composition, organization, and structure by providing both intra- and extracellular spatial information through sensitivity to Brownian motion of water molecules1,2. Advanced DWI methods are gaining momentum as powerful diagnostic and research tools in the spinal cord, giving their sensitivity to tissue microstructure beyond conventional MRI measurements. Although quantitative MRI (qMRI) techniques such as DWI provide metrics that are quantitative by construction and should be reproducible across hardware/software configurations, scanner upgrades can introduce a systematic bias in qMRI3,4. There are many concerns in the context of multi-centre and/or longitudinal studies about reliability, possible bias, and reproducibility of quantitative measurements1,3. This study aimed to characterise the effect of a major upgrade at a single site and quantitatively compare diffusion metrics obtained in the spinal cord. The upgrade consisted in updating a 3T Philips Achieva system to the software and hardware of a 3T Philips Ingenia CX scanner, including a digital broadband receive system architecture.Methods
Participants: Four healthy controls (1 female) were scanned before and after the upgrade of the 3T MRI system (Philips Healthcare, Best, The Netherlands).MR imaging: The 3T MRI system (Philips Healthcare, Best, The Netherlands) was upgraded from the Achieva to the Inginia CX platform. The imaging protocol included a 3D gradient-echo with in plane resolution= 0.5 x 0.5 mm2, slice thickness = 5 mm, TR = 23 ms, TE = 5 ms, flip angle = 7 degrees, FOV = 240 x 240 mm2 and a DWI protocol, which consisted of 72 volumes distributed as 9 (b= 0s/mm2), 21 (b= 1000 s/mm2), 21 (b= 2000 s/mm2), 21 (b= 3000 s/mm2). The post-upgrade scans included an additional volume at b=1000 s/mm2. A small FOV multi-slice single shot-EPI readout, with outer-volume-suppression was used, with 1x1x5 mm3 resolution, FOV = 64x48 mm2, triggered to cardiac cycle via a peripheral pulse unit. All images were centred at C2/C3 inter-vertebral disc, covering 6cm of the cervical spinal cord.
Image analysis: The same automatic image processing pipeline was applied to the pre-/post-upgrade data, without user intervention. DWI volumes were denoised using random matrix theory and noise floor mitigated5,6. Motion within the DWI protocol was corrected using slice-wise translation in the axial plane, regularized along the z direction6,7. Quantitative diffusion metrics from diffusion tensor and kurtosis imaging (DTI and DKI)1,2, as well as neurite orientation dispersion and density imaging (NODDI)8 were computed from motion-corrected data, using open-source software9,10. The 3D gradient-echo data was used to automatically identify spinal cord regions-of-interest (ROI) for the downstream analysis, to avoid potential bias introduced. Whole-cord, white matter (WM), and grey matter (GM) ROIs were obtained using the spinal cord toolbox (SCT) deep-learning segmentation tool11,12, and registered to the diffusion data via non-rigid transformation. Here we report the effect of the system upgrade on a subset of the calculated metrics, fractional anisotropy (FA), mean diffusity (MD), mean kurtosis (MK) and neurite density index (NDI).
Statistical analysis: Bland-Altman plots were used to represent the mean differences between the upgrades in the ROIs. Confidence intervals were reflected by limit agreement, with 95% of the differences between pre-/post-upgrade metrics estimated by the mean difference ± 1.96 times the standard deviation (SD) of the differences13. Coefficient of variation (%CV)14 provides an estimate of the amount of variability within the group, with respect to the mean population value of the subject.
Results
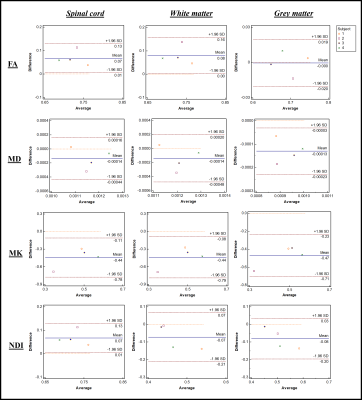
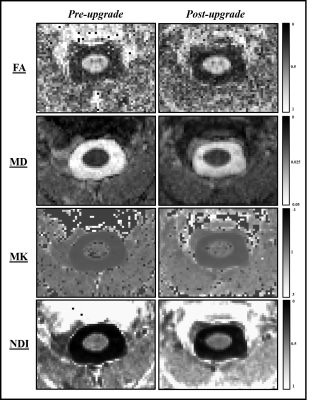
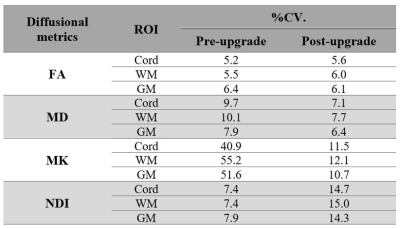
Bland-Altman plots for four subjects for each ROI and for four diffusion metrics are shown in Fig. 1. From these plots, the mean differences are 0.07 (FA), -0.0014 (MD), -0.44 (MK) and 0.07 (NDI). In WM they are 0.08 (FA), -0.0014 (MD), -0.44 (MK) and 0.07 (NDI) and in GM 0 (FA), -0.0013 (MD), -0.47 (MK) and -0.08 (NDI). The mean of the differences in the diffusion metrics are all contained within the 95% confidence intervals for all ROIs. Parametric maps for FA, MD, MK, and NDI are represented in Fig. 2, with minor differences detectable by visual inspection. Table 1 shows stable reproducibility figures for pre- and post-upgrade, expressed as the %CV.Discussion and Conclusion
We aimed to assess the impact of a major MRI system upgrade by performing a direct comparison of spinal cord diffusion metrics using DWI from four subjects in three ROIs. Bland-Altman plots can be used to estimate agreement between pre- and post-upgrade metrics, as they provide a graphical display of bias (mean difference between upgrades) with 95% limits of agreement. The diffusivity metrics from DTI and DKI do not vary substantially between pre- and post-upgrade, with the bias of each of these metrics found to only deviate slightly from the mean value. To assess repeatability, %CV were analysed showing a comparable variability. A greater variability is found in multi-compartment models parameters such as NODDI. This confirms that the diffusional metrics (FA, MD, and MK) can be measured with similar or improved precision post-upgrade. Interestingly, NDI shows a higher inter-scan reproducibility pre-upgrade than post-upgrade and merits further investigation. It is therefore highly recommended that pre- and post-upgrade data is always collected, especially for studies that are recruiting subjects across the upgrade, preferably using larger sample size, to verify qMRI metrics reproducibility.Acknowledgements
The UK MS Society and the UCL-UCLH Biomedical Research Centre for ongoing support. CGWK receives funding from the UK MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541. We thank Philips Healthcare for assistance in protocol development and for access to research protocols. FG is currently supported by the investigator initiated PREdICT study at the Vall d'Hebron Institute of Oncology (Barcelona), funded by AstraZeneca. RB is supported by the Chiang Mai University, Thailand for studentship funding.
References
- Tofts P. Quantitative MRI of the brain: measuring changes caused by disease, John Wiley & Sons. 2005.
- Le Bihan D. Du mouvement Brownien aux images de la pensée: l'IRM de diffusion [From Brownian motion to mind imaging: diffusion MRI]. Bull Acad Natl Med. 2006 Nov;190(8):1605-27; discussion 1627. French. PMID: 17650747.
- Shaw C, Jensen J, Deardorff R, et al. Comparison of Diffusion Metrics Obtained at 1.5T and 3T in Human Brain With Diffusional Kurtosis Imaging. J Magn Reson Imaging. 2017 Mar;45(3):673-680. doi: 10.1002/jmri.25380. Epub 2016 Jul 12. PMID: 27402163.
- Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177-92.
- Jelle V, Dmitry S N, Daan C, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage. 142 (2016): 394-406.
- Francesco G, Marco B, Jelle V, Torben S, et al. Multi-parametric quantitative in vivo spinal cord MRI with unified signal readout and image denoising. Neuroimage (2020): 116884.
- Benjamin D L, Simon L, Sara M D, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 145 (2017): 24-43.
- Francesco G, Torben S, Hui Z, et al. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord in vivo. Neuroimage. 111 (2015): 590-601.
- Eleftherios G, Matthew B, Bagrat A, et al. Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics. 8 (2014): 8.
- Rutger F, Demian W, and Rachid D. The dmipy toolbox: Diffusion mri multi-compartment modeling and microstructure recovery made easy. Frontiers in neuroinformatics. 13 (2019): 64.
- François P, Jennifer L, Christian S P, et al. Open-source pipeline for multi-class segmentation of the spinal cord with deep learning. Magnetic Resonance Imaging. 64 (2019): 21-27.
- Christian S P, Evan C, and Julien C. Spinal cord gray matter segmentation using deep dilated convolutions. Scientific reports. 8, no. 1 (2018): 1-13.
- Bland J, Altman D. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160.
- Bland J M, Altman D G. Measurement error. BMJ: British medical journal. 1996a;312: 1654.
Figures