2453
Influence of electrocardiogram signal triggering on filter exchange imaging1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Synopsis
Apparent exchange rate (AXR) mapping can be used to non-invasively investigate water exchange between the intra- and extracellular compartment, which might yield insight into cell membrane permeability. To obtain the AXR properly with the filter exchange imaging (FEXI) approach, signal stability is crucial. We show that electrocardiogram (ECG) triggering can lead to a slight improvement of the signal stability for AXR measurements in the human brain. However, pulsation-induced fluctuations are not the main source of signal variations. Thus, it remains questionable if investing additional measurement time in triggering is justified.
Introduction
The permeability of cell membranes is an important biological parameter, which might be changed in pathologies. Recently, apparent exchange rate (AXR) mapping has been introduced, which might allow gaining access to this quantity non-invasively [1,2]. In the context of imaging, optimum experimental parameters for the human brain have been published [3]. However, the technique suffers from intrinsically low signal-to-noise ratio as a result of the two diffusion weightings and the use of long exchange times during which T1 relaxation occurs. Pulsation is known to affect DWI in the brain and may further degrade signal quality [4]. Therefore, similar filter exchange imaging (FEXI) experiments were carried out using electrocardiogram (ECG) signal triggering. In this study, we analyzed the obtained FEXI signals with respect to stability.Methods
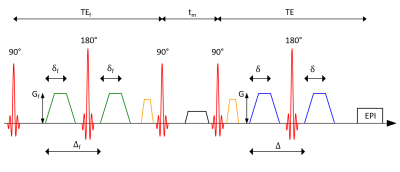
Experiments were carried out on a SIEMENS 3T Prisma imaging system. A schematic representation of the used FEXI sequence can be found in Figure 1. Data were obtained from one healthy volunteer who gave written informed consent beforehand. Three axial slices of the brain with a spatial resolution of 3×3×5 mm3 were acquired with a 64-channel head coil. To investigate the influence of the ECG triggering on the filter exchange imaging approach, three mixing times $$$\mathrm{t_m}$$$ = [50, 200, 350] ms were used. For each mixing time, the $$$\mathrm{b}$$$-value of the filter was set to 900 s/mm2. Timing parameters in the filter block were $$$\mathrm{\delta_f}$$$ = 9.3 ms and $$$\mathrm{\Delta_f}$$$ = 14.6 ms resulting in a filter echo time of $$$\mathrm{TE_f}$$$ = 35 ms. Two $$$\mathrm{b}$$$-values of 0 s/mm2 and 900 s/mm2 were applied for diffusion encoding. The durations of the diffusion-weighting gradients were constant at $$$\mathrm{\delta}$$$ = 9 ms, time difference between the gradient onsets was $$$\mathrm{\Delta}$$$ = 15.3 ms.The maximum gradient strength in both filter and diffusion encoding block was $$$\mathrm{G}$$$ = 75 mT/m. Three orthogonal diffusion encoding directions were used: ($$$\mathrm{g_x}$$$, $$$\mathrm{g_y}$$$, $$$\mathrm{g_z}$$$) = (2/3, 2/3, 1/3), (1/3, 2/3, 2/3) and (2/3, 1/3, 2/3), where $$$\mathrm{g_i}$$$ indicates the normalized strength of the diffusion-weighting gradients along the scanner axes $$$\mathrm{x}$$$, $$$\mathrm{y}$$$ and $$$\mathrm{z}$$$, respectively.
Four repetitions with equal parameters were acquired. The receiver bandwidth was set to 2000 Hz/ pixel and a phase partial Fourier factor 5/8 was used. The resulting echo time in the diffusion encoding block was $$$\mathrm{TE}$$$ = 42 ms in all acquisitions, the repetition time was set to 2800 ms.
Triggering was done on the cardiac R wave with an acquisition window of 3400 ms. One dataset was obtained without triggering, three datasets were acquired with trigger delay times $$$\mathrm{T_d}$$$ = [0, 200, 400] ms for the trigger. To assess the influence of the ECG triggering, the standard deviation of the signal in each voxel of a slice over the four repetitions was calculated. The standard deviation was averaged over the voxels chosen by a signal threshold on $$$\mathrm{b}$$$ = 0 s/mm².
Results
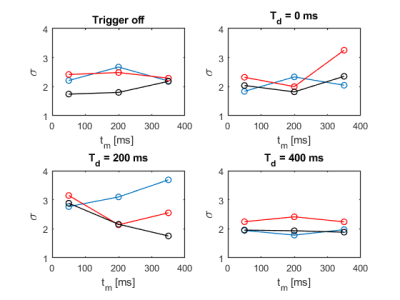
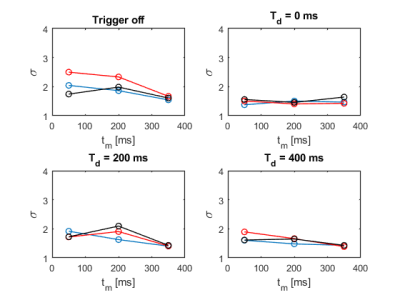
For each used $$$\mathrm{b}$$$-value in the diffusion encoding block, Figure 2 and 3 display the obtained standard deviations for each performed trigger experiment in dependency of the mixing times, respectively. The three orthogonal diffusion directions are shown separately.Figure 2 shows the standard deviation of the signal for different trigger delays $$$\mathrm{T_d}$$$ when the filter weighting is turned on while the actual diffusion encoding is turned off. No large changes in the standard deviation can be observed; a trend towards reduced stability seems to be present for $$$\mathrm{T_d}$$$ = 200 ms, while $$$\mathrm{T_d}$$$ = 400 ms appears to be performing best for this experiment.
When also diffusion weighting is turned on, ECG triggering seems to lead to slightly improved signal stability for all trigger delays, while $$$\mathrm{T_d}$$$ = 200 ms again appears to be performing worst. $$$\mathrm{T_d}$$$ = 0 ms and $$$\mathrm{T_d}$$$ = 400 ms exhibit better results.
Discussion and Conclusion
It could be shown that ECG triggering has an influence on signals acquired with a FEXI sequence in the brain of a healthy volunteer. Signals obtained with low mixing times might be generally more stable when the imaging sequence begins at the end of the diastole, i.e. $$$\mathrm{T_d}$$$ = 0 ms. Depending on the mixing time, using long trigger delay times may also be a viable option. The preliminary nature of this study including only one volunteer has to be noted.In general, the change of the standard deviation when enabling ECG triggering remains rather low, so that it appears that other sources of noise are dominant contributors to signal fluctuations in AXR measurements. Furthermore, triggering generally increases measurement time. Therefore, it remains questionable, whether investing additional measurement time for triggering is justified and need to be clarified by a more detailed analysis.
Acknowledgements
Financial support by the DFG (Grant No. KU 3362/1-1) is gratefully acknowledged.References
[1] Lasič S et al. Apparent exchange rate mapping with diffusion MRI. Magn Reson Med. 2011;66(2)
[2] Nilsson M et al. Noninvasive mapping of water diffusional exchange in the human brain using filter‐exchange imaging. Magn Reson Med. 2013;69(6)
[3] Lampinen B et al. Optimal experimental design for filter exchange imaging: Apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magn Reson Med. 2017;77(3)
[4] Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53(5)
Figures