2450
Rapid Multi-slice STEAM Diffusion Imaging with a Prepared Gradient Echo Echoplanar Sequence1Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Global MR applications and workflow, GE Healthcare, Boston, MA, United States
Synopsis
STEAM diffusion offers longer diffusion times, reduced gradient demands and other advantages for diffusion imaging but has suffered from long acquisition times. We propose a non-selective preparation of a conventional gradient echo echoplanar sequence that enables the rapid acquisition of many slices. Initial application to the brain readily enabled up to 32 slice acquisition within a single TR.
Introduction
Stimulated echo (STEAM) diffusion imaging1-3 offers a number of advantages over spin echo diffusion imaging. Because of the slower, T1 decay during the TM time, STEAM can probe a wide range of diffusion times and achieve very large diffusion weightings with modest gradient amplitudes and duty cycles. STEAM also offers reduced T2 sensitivity and greater suppression of short T1 fat than spin echo imaging. Finally, cardiac gating of STEAM gradients can be used to virtually eliminate cardiac motion related signal loss that is otherwise a problem in myocardium3, regions of the liver1, and even the spinal cord2. Past acquisitions across large volumes with STEAM suffer from slow acquisition times because a limited number of slices could be acquired for each STEAM TM period. This is particularly a limitation now that multi-band spin echo diffusion imaging can be used to acquire many dozens of slices within a few seconds. In this work, we highlight how a non-selective STEAM preparation can be combined with a modified multi-band and multi-slice gradient echo EPI acquisition to acquire a large number of diffusion encoded slices in a short time. Though nonselective STEAM preparation has been employed for one or a few slices in cardiac diffusion imaging, the potential for large volume acquisition has not previously been appreciated. The non-selective preparation enables a number of simple options for fat suppression and reduced field of view imaging, that may be of particular value in spinal cord and body applications. A strategy to correct for differences in diffusion weighting across slices is proposed.Methods
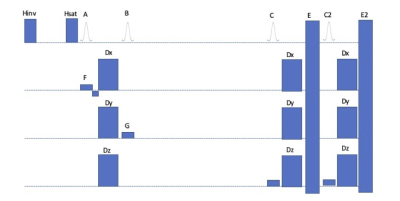
The sequence was implemented on a 3T scanner (GE MR750) as a modification to a conventional gradient echo echoplanar sequence(figure 1). A preparation module consisting of 2 nonselective 90° pulses initiates the STEAM sequence. These pulses can be made weakly selective (e.g. F,G) to conveniently define reduced fields of view for the acquisition if desired. Optional fat saturation (Hsat) or spectrally selective or nonselective STIR fat suppression (Hinv) pulses can be employed. Diffusion gradients are added between the two 90’s. Readout is achieved by a rapid series of gradient echo echoplanar acquisitions with the simple addition of diffusion and crusher gradients to refocus the stimulated echo. Multiband RF pulses and reconstruction are readily supported. Because successive slices are acquired at different times, the change in TM results in a change of b value and T1 decay. If uniformity of intensity is a priority or the number of slice excitations is large, correction of the decay can be achieved by acquiring two sets of images with acquisition order reverse. Taking the geometric mean of the two images for each slice makes T1 and diffusion weighting uniform across slices.Results
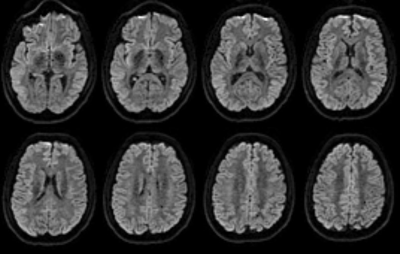
The sequence was evaluated in phantoms and for brain imaging in two healthy volunteers. b=1000 s/mm2 images were acquired with a diffusion time of 1s and an isotropic spatial resolution of 2.5mm. A TR of 3s, the two acquisition orders and 4 repetitions were performed. Both non-multiband 8 slice acquisitions and 30 slice acquisitions with 6x multiband were acquired. Good image quality and successful compensation of differential diffusion and T1 weighting were achieved.Conclusions
The use of nonselective preparation overcomes many of the challenges of STEAM diffusion imaging across large volumes. This approach shows promise for applications where long diffusion times, high diffusion weighting, gating to remove cardiac pulsations, improved fat suppression, reduced fields of view and/or minimizing gradient duty cycles are needed.Acknowledgements
NoneReferences
1. Muller MF et al. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology 1994;190(2):475-8
2. Rangwala NA et al. Diffusion restriction in the human spinal cord characterized in vivo with high b-value STEAM diffusion imaging. Neuroimage. 2013;82:416-25
3. Edelman RR et al. In vivo measurement of water diffusion in the human heart. Magn Reson Med 1994;32(3):423-8