2443
Accelerating Brain Diffusion Tensor Imaging using Neural Networks: A Comparison of three Neural Networks1Medical Physics Graduate Program, Duke University, Durham, NC, United States, 2Department of Radiation Oncology, Duke University, Durham, NC, United States
Synopsis
This work focused on accelerating DTI using deep learning methods. Three neural networks including U-net, PD-net and Cascade-net were investigated on reconstructing DTI images, ADC maps and FA maps from Cartesian under-sampled k-space data. The results indicated that Cascade-net out-performed the other two networks, obtaining comparable image quality as compared with the reference reconstructed from full k-space data. In summary, neural networks can be used to accelerate DTI while maintaining image quality.
Target audience
Clinicians, physicists and other clinical professionalsPurpose
Magnetic Resonance Imaging (MRI) obtains a great discernibility of soft tissue, thus considered as a great tool for diagnosis and treatment. However, the acquisition process can be slow due to its physical nature compared with other modalities, which would reduce its effectiveness on clinical application. Diffusion Tensor Imaging (DTI) would consume even longer time since it requires multiple diffusion weighted (DW) acquisitions for encoding diffusion information along different directions. To overcome the limit, deep learning methods have been proposed to accelerate the MRI, where magnetic resonance (MR) images are reconstructed from under-sampled k-space data using well-trained neural network models. While many works have been reported to accelerate standard anatomic MRI,1-2 few studies are reported on DTI. In this work, three neural networks including U-net, Primal-Dual-net (PD-net) and Cascade-net were investigated on accelerating brain DTI, and their performances were compared, quantitatively evaluated using Total Relative Error (TRE).Methods
DTI: In DTI, the eigenvalues of a 3-by-3 symmetric diffusion tensor matrix, denoted as λ1, λ2 and λ3, are calculated to quantify the water diffusion along each direction. The Apparent Diffusion Coefficient (ADC) and Fractional Anisotropy (FA) can be calculated as3$$ADC=D_{av}=\frac{(\lambda_1+\lambda_2+\lambda_3)}{3}$$
$$FA=\sqrt{\frac{3}{2}}\frac{\sqrt{(\lambda_1-D_{av})^{2}+(\lambda_2-D_{av})^{2}+(\lambda_3-D_{av})^{2}}}{\sqrt{\lambda_1^{2}+\lambda_2^{2}+\lambda_3^{2}}}$$
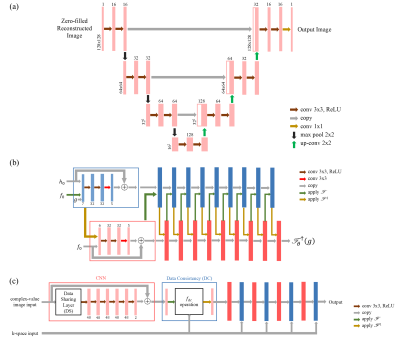
Neural Networks: Three neural networks: U-net, PD-net and Cascade-net were evaluated in this work. U-net is a single-domain network that only acts on either k-space data or image space data. It was firstly proposed by Ronneberger et al.4 for image segmentation purpose and now serves as a strong baseline in image processing. PD-net and Cascade-net can be defined as cross-domain networks where both k-space data and image space data are used during training process. The structures of the three networks are illustrated in Figure 1.a, 1.b and 1.c respectively.1-2, 4-5
Training and Test of Neural Networks: A Cartesian sampling strategy was adopted with an acceleration factor of 4 (i.e., 25% of the k-space data was sampled), illustrated as Figure 2. The central 8% of k-space data was kept, and the other 17% of k-space data was randomly sampled in the periphery following uniform distribution. The training data including 381 brain DTI sets are obtained from the RIDER NEURO MRI dataset.6 Among the training data, 312 brain DTI sets were used for training, and the remaining 69 brain DTI sets were used for validation. All the models were trained for 15 epochs using Tensorflow framework. One independent in vivo brain DTI study with 12 tensor directions (b value = 1000 sec/mm2) was used for test and evaluation of three different neural networks. In this work, TRE was used to assess the quality of reconstructed images, which is defined as7
$$TRE=\frac{\sqrt{\sum_{x,y}[M_0(x,y)-M_g(x,y)]^2}}{\sum_{x,y}M_g(x,y)}$$
Results
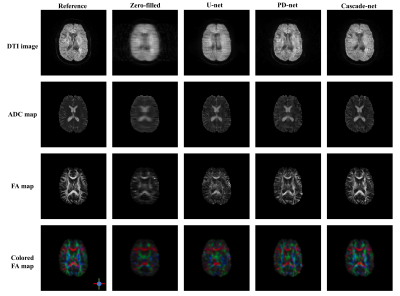
With an acceleration factor of 4, the reconstructed images by Cascade-net generally showed comparable to the reference images reconstructed from full k-space data and superior over those by U-net and PD-net, as shown in Figure 3. As measured by TRE values, Cascade-net showed better performance than U-net and PD-net on the reconstruction of DTI images, ADC and FA maps. Although PD-net and U-net obtained similar performance on DTI image reconstruction, PD-net out-performed U-net on the reconstruction of ADC and FA maps, as summarized in Table 1. It should be noted that the image quality of images as measured by TRE declined from reconstructed DTI images to quantitative ADC and FA maps. This finding might suggest that the error in reconstructed DTI images would be amplified in ADC maps and even more in FA maps.Discussion and Conclusion
In this work, three neural networks: U-net, PD-net and Cascade-net were investigated on accelerating brain DTI. Among the three neural networks, the Cascade-net showed the best performance on reconstruction of DTI images and quantitative functional diffusion maps. This work could be further improved by optimizing the neural network structures and by adopting non-Cartesian sampling strategies. As a general method, the fast DTI MRI using neural networks could be extended to a different clinical site such as spine.Acknowledgements
This work is financially supported by Duke Cancer Institute.References
1. Ramzi Z, Ciuciu P, Starck J-L. Benchmarking MRI Reconstruction Neural Networks on Large Public Datasets. Applied Sciences. 2020; 10(5):1816.
2. Schlemper J, Caballero J, Hajnal JV, Price AN, Rueckert D. A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging. 2018;37(2):491-503
3. Kingsley PB. Introduction to diffusion tensor imaging mathematics: Part II. Anisotropy, diffusion-weighting factors, and gradient encoding schemes. Concepts Magn Reson Part A Bridg Educ Res. 2006;28A (2):123-154.
4. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In: Lecture Notes in Computer Science. Springer International Publishing; 2015:234-241.
5. Adler J, Oktem O. Learned Primal-dual reconstruction. IEEE Trans Med Imaging. 2018;37(6):1322-1332.
6. Barboriak, Daniel. (2015). Data From RIDER_NEURO_MRI. The Cancer Imaging Archive. http://doi.org/10.7937/K9/TCIA.2015.VOSN3HN1
7. Chang Z, Xiang Q-S, Ji J, Yin F-F. Efficient multiple acquisitions by Skipped Phase Encoding and Edge Deghosting (SPEED) using shared spatial information: Efficient Multiple Acquisitions by SPEED. Magn Reson Med. 2009;61(1):229-233.
Figures