2434
MRI-based deep learning model in differentiating benign from malignant renal tumors: a comparison study with radiomics analysis1Northern Jiangsu People's Hospital, Yangzhou, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
The aim of this study was to evaluate the feasibility of magnetic resonance imaging (MRI) based deep learning (DL) model in differentiating benign from malignant renal tumors. The performance of the applied DL model was further compared with that from a random forest radiomics model. More robust performance was achieved using MRI based DL model than the radiomics model (AUC = 0.925 vs 0.854, p<0.05). Therefore, the applied MRI based deep transfer learning model might be considered a convenient and reliable approach for differentiating benign from malignant renal tumors in clinic.
Introduction
Approximately 20% of surgically removed renal masses are benign, indicating unnecessary surgical removal of benign tumors in many clinical cases.1 As the imaging characteristics of benign renal tumors are similar to renal cell carcinoma (RCC), preoperative diagnosis is difficult using conventional MR imaging.Magnetic resonance imaging (MRI) based computer-assisted methods might be able to identify subtle morphological differences between benign and malignant tumor groups, and thus hold the potential to overcome this issue.2 Radiomics model, as a computed-assisted method, can extract and integrate imaging features quantitatively, and has been applied to classify renal tumors.3
Meanwhile, deep learning (DL) model provides a new classification strategy based on artificial intelligent pattern recognition of images, without relying on predefined metrics.4 Structural MRI information has been applied in DL analysis for differentiating benign from malignant renal tumors.5 Diffusion weighted imaging (DWI) is a functional MRI technique that characterizes tissues by their water diffusion properties, we hypothesized that combining anatomical features (T2WI) and functional imaging (DWI) might achieve improved performance for renal tumor differentiation. No study has however investigated this.
Therefore, the main goal of this study was to explore the feasibility of T2WI and DWI combined MRI based DL model in differentiating benign from malignant renal tumors by comparing with a radiomics model.
Methods
Subjects286 patients with histopathologically confirmed RCC and benign tumor (oncocytoma and angiomyolipoma) were recruited in this study.
MR experiments
All patients were examined with a 3-T MRI (GE750, Milwaukee, WI, USA) using an eight-channel array body coil. T2WI anatomic imaging was acquired with the scan parameters of 24 axial slices covering both kidneys; DWI used a single-shot spin-echo-echo-planar imaging (SE-EPI) sequence with a b value of 800 s/mm2. Detailed acquisition parameters are listed in Table 1.
Data analysis
All T2WI and DWI data were exported to Darwin intelligent scientific research platform. The region of interest (ROI) was manually outlined by a senior radiologist in abdominal imaging. The workflow of applied DL and radiomics model is presented in Fig.1.
Radiomics model
Radiomics features were extracted from both T2WI and DWI images of each patient using Pyradiomics 2.1.0. The multivariate logistic analysis and selection operator (LASSO) regression method were used for feature selection, and the radiomics model was built by random forest classifier method for discrimination ability.
Deep Learning model
ROI patches were automatically extracted from each MRI image. A bounding box was created to completely enclose the ROI. Three pixels of surrounding features were retained on the four directions (up, down, left and right), and all images were then resized to 224 × 244 × 3. Each pixel was first normalized into the range of 0 to 1 and then input to the network. Due to limited training data, we applied random flipping and random rotation procedures for training data augmentation.
In this study, a ResNet-18 network pre-trained on the ImageNet images was applied.6 This DL model used combined T2WI and DWI images as input. The model with input DWI converged after 5 iterations, and with input T2WI converged after 14 iteration. The output was the classification of benign vs. malignant renal tumors. During training, Adam optimization with a decay of 1*10-4,batch size of 4, an initial learning rate of 5*10-5 were applied. Learning rate was decayed to the ninth power of iterations number. During testing, the prediction results from both input were averaged and integrated as the final prediction.
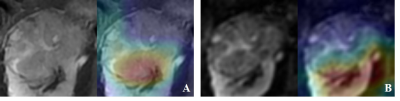
To further understand the applied DL model for our prediction task, a DL visualization technique called Class Activation Map was used to produce a heat map of class activation over input images.
Statistical analysis
All statistical analyses were performed using R version 3.4.3. Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the predictive performance of radiomics or DL model. The comparison of ROC curves was performed by Delong-test. P<0.05 was considered significant.
Results
In total 160 RCCs and 57 benign tumors were recruited, and divided into two cohorts: a primary cohort (n = 173) and a validation cohort (n = 44). The primary cohort consisted of 45 benign and 128 malignant tumors, and the validation cohort included 12 benign and 32 malignant tumors.Using ROC analysis, DL has shown a better diagnostic efficiency than radiomics (AUC = 0.925 vs 0.854,p<0.05). Detailed ROC relevant results are shown in Table.2.
The classification activation maps were shown for both T2WI and DWI (Fig.2). Tumoral and peri-tumoral area were highlighted, being valuable for feature pattern extraction.
Discussion and Conclusion
To our knowledge, this is the first attempt to test the feasibility of an anatomical and functional combined MRI-based DL approach in differentiating benign from malignant tumors. Our results showed that the ResNet-18 model showed superior performance in tumor differentiation to radiomics model. In addition, the generated feature maps from ResNet-18 model provided additional spatial heterogeneity on tumor area, indicating the ability of deep learning to discover spatial heterogeneity of a tumor.In conclusion, the applied MRI-based deep transfer learning model might be considered an effective tool for differentiating benign from malignant renal tumors in clinic.
Acknowledgements
None.References
1. Fujii Y, Komai Y, Saito K, et al. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology. 2008;72(3):598–602
2. Matsuo H, Nishio M, Kanda T, et al. Diagnostic accuracy of deep-learning with anomaly detection for a small amount of imbalanced data: discriminating malignant parotid tumors in MRI. Sci Rep. 2020; 10(1): 19388.
3. Said D, Hectors J, Wilck E, et al. Characterization of solid renal neoplasms using MRI-based quantitative radiomics features. Abdom Radiol. 2020; 45(9): 2840-2850.
4. Afshar P, Mohammadi A, Tyrrell N, et al. deep learning-based radiomics for the time-to-event outcome prediction in lung cancer. Sci Rep. 2020; 10(1): 12366.
5. Xi I, Zhao Y, Wang R, et al. Deep Learning to Distinguish Benign from Malignant Renal Lesions Based on Routine MR Imaging. Clin Cancer Res. 2020; 26(8): 1944-1952.
6. Kaiming H, Xiangyu Z, Shaoqing R, et al. "Deep residual learning for image recognition." Proceedings of the IEEE conference on computer vision and pattern recognition. 2016.
Figures